As FDA Remains Mum on Stent Probe, Market Decline Continues
October 1, 2007
NEWS TRENDS
|
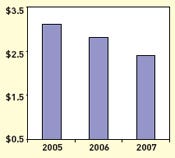
Annual sales of drug-eluting coronary stents have fallen from $3.1 billion to $2.4 billion in the past two years. |
An investigation launched by a U.S. House of Representatives committee is the latest in a series of problems faced by drug-eluting stent companies in the past year. This most recent bump in the road is a probe by Congress into how FDA handled issues related to Johnson & Johnson's (New Brunswick, NJ) stent manufacturing practices nearly four years ago.
The inquiry is driven by John Dingell (D-MI), chairman of the House Committee on Energy and Commerce, and Bart Stupak (D-MI), chairman of the Subcommittee on Oversight and Investigations. They sent separate letters in August to FDA Commissioner Andrew von Eschenbach and William Weldon, CEO of Johnson & Johnson. According to the letter to von Eschenbach, agency inspectors found “numerous systemic violations” related to the manufacture of the Cypher stent during visits to Cordis Corp. facilities in 2003.
“Despite these numerous violations, however, Cordis was allowed to continue marketing Cypher stents,” wrote Dingell and Stupak. FDA declined to comment on the letters.
Following postapproval inspections of Cordis Corp.'s plants, FDA sent a warning letter to the company in April 2004. It cited a range of concerns. Among them: not establishing corrective and preventative actions and failure to validate processes adequately. FDA lifted the warning letter this past June, noting that the problems named in the letter had been resolved.
Despite the resolution of the warning letter, Dingell and Stupak are asking for all records related to the warning letter and inspection reports. They also want all communication, including e-mail and meeting notes, from FDA staff connected to the Cordis and Cypher inspections that occurred between July 2003 and April 2004. The letter sent to Weldon requests all internal J&J communication related to Cordis and Cypher inspections between September 2003 and June 2007.
|
Rajan says stent investigations in the United States will affect global sales. |
The investigation is the latest in a series of troubles for drug-eluting stents and is expected to hit the already declining stent market. “Anything that happens around the world will have a ripple effect,” says Venkat Rajan, industry analyst at Frost & Sullivan (San Antonio, TX). “Here in the United States, especially if there's a congressional investigation into manufacturing processes, it will definitely impact the market.”
The drug-eluting stent market took a dip last year, from $3.1 billion in 2005 to about $2.8 billion in 2006. According to Rajan, the market is expected to drop further to about $2.4 billion in 2007. He attributes part of this decline to the Courage study results, which revealed that drug-eluting stents were no more effective than their bare-metal counterparts in preventing heart attacks and death in patients with coronary artery disease.
Research on drug-eluting stents has also suggested that the devices could raise the risk of blood clots after implantation, and pose a higher risk of complications when they are used off-label. These factors have also played a role in how the market has fared.
A similar decline overseas could get worse. At press time, the British government was considering a proposal to end reimbursement for drug-eluting stents, citing safety concerns. If implemented, the policy would force patients to receive bare-metal stents or undergo alternative treatments if they do not want to pay out of pocket. British and European cardiology societies have been fighting the proposal vigorously.
However, Rajan points out that drug-eluting stents are still a useful and life-saving technology and won't be abandoned by doctors. “There's been so much hype about the product that market participants and investors overestimated the potential of the devices. When you say market decline or market slump, it's in relation to what investors have been expecting with this product.”
For now, the market is expected to steadily grow, mainly due to high patient demand and the time crunch in hospitals that make stenting more convenient than open-heart procedures. Rajan doesn't anticipate a jump similar to what happened in the early 2000s unless a next-generation device such as a bioabsorbable stent is introduced.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)