The Role of Clinical Studies in Imaging Validation
COVER STORY Focused for Growth
January 1, 2007
Clinical trials play a central role in evaluating the safety and efficacy of many therapeutic products, including pharmaceuticals as well as medical devices. But such studies are also valuable when it comes to assessing the utility of diagnostic strategies made possible by the latest generations of medical imaging technologies. Today, companies in the medical imaging sector are embracing the use of clinical trials as a critical obligation for bringing new products to market.
|
|
|
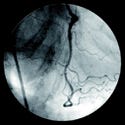
Toshiba's CorE 64 clinical trial compares the clinical benefits of coronary CT angiography (top) with cardiac catheterization (bottom). |
Clinical trials require a huge investment from the supporting company as well as the physician partners conducting the study. But they also offer a significant payoff. Such studies can help to establish the clinical utility of a technology for viewing and diagnosing conditions that were previously impossible to visualize. Often, clinical trials can enable investigators to determine which patient populations will benefit most from the use of a new technology or clinical application. And clinical studies also play a significant role in measuring productivity increases and verifying techniques that reduce healthcare costs. In today's healthcare environment, all of these purposes are important for determining the viability of a new technology.
Several factors are contributing to the adoption of this new strategy in medical imaging. The sector is experiencing rapid growth due to improvements in image resolution and speed of acquiring images. In addition, the uses for medical imaging are expanding beyond radiology and becoming critical to patient treatment in such specialty areas as cardiology, emergency medicine, and oncology. Despite the importance of new imaging technologies to healthcare providers, there is growing recognition that such technologies and their associated procedures must be validated against gold standards of care if they are to be adopted into routine clinical use.
In 2004, working with partners around the world, Toshiba launched its coronary evaluation on 64-slice CT (CorE 64) study, the industry's first multicenter clinical study to investigate the use of 64-row multislice computed tomography (CT) as the primary diagnostic tool for the detection of cardiovascular diseases and disorders. Using Toshiba's Aquilion 64, the study will evaluate whether today's CT technology could replace diagnostic cardiac catheterization as the gold standard in cardiac imaging.
The CorE 64 investigating partners include Johns Hopkins University School of Medicine (Baltimore); Beth Israel Deaconess Medical Center, Harvard Medical School (Boston); Humboldt University, Campus Charite Mitte (Berlin); Iwate Medical University (Iwate, Japan); Toronto General Hospital, University Health Network (Toronto); and Mount Sinai Hospital (Toronto). More than 300 patients have been enrolled, and results are expected sometime in 2007.
Copyright ©2007 MX
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

