Are you the master of your data?
April 11, 2017
Enterprise labeling systems can help medical device manufacturers manage data in some surprising ways.
Enterprise labeling software solutions have been helping medical device manufacturers manage label design, printing, and more for years. But in today's data-rich environment thanks to Unique Device Identification, pharma serialization, and wearable devices, are such systems delivering all the necessary functionality and cybersecurity? Qmed asked Ardi Batmanghelidj, President and CEO of Innovatum, a software and regulatory consulting company specializing in life science labeling and Unique Device Identification (UDI) compliance, for an update on labeling software for the medical device, pharma, and biotech industries.
|
Ardi Batmanghelidj, President and CEO of Innovatum |
Qmed: How are enterprise labeling systems being used today by medical device companies?
Batmanghelidj: Enterprise labeling systems (ELS) have evolved past being merely a means of placing data on labels efficiently and complying with 21 CFR (title 21 of Code of Federal regulations). Such systems are capable of much more than they were in the past. An ELS can now serve as the glue that makes data reuse consistent across labeling, regulatory submissions, electronic IFU management, 100% print inspection, etc. The belief that printing data on the label is the end of the ELS story is now quickly ending. Multifunctionally applicable data can now be exploited across varied yet closely related labeling disciplines, thereby guaranteeing synchronization of data, data accuracy, and validity. Medical device companies have been seeking this level of optimization for quite some time, and now it is at hand.
Qmed: Are such systems being used for their full potential?
Batmanghelidj: The ability to use any system to its full potential over an extended period is ultimately determined by the flexibility that is inherent within that system. Software is unique in its ability to create opportunities to paint oneself into a corner. When a system is designed myopically with limited functional intentions, the usable life of the system makes itself evident over time as needs evolve. Use of a "COTS" (commercial off the shelf) labeling system provided by software specialists who benefit from a consortium of customer inputs and industry involvement ensures a living, growing, and adaptable system. Although many life sciences companies are using their enterprise labeling systems to their full potential, the potential of these systems is often limited in scope.
Qmed: Are there any features or functions that would surprise medtech professionals?
Batmanghelidj: An immediate focus upon the need to bail quickly often detracts from the thought of finding something to plug a leak. There are many breakthroughs in enterprise labeling that are rapidly creating paradigm shifts from traditional labeling approaches. These breakthroughs represent opportunities for significant reductions in risk and cost and greatly improved efficiency and productivity. Life sciences-specific master data management, mass label changes, automated eIFU hosting, automated 100% print inspection, and native integration with product life cycle management (PLM) systems like Agile constitute the most notable developments of late.
Qmed: What regulatory drivers are there for employing enterprise labeling software?
Batmanghelidj: Don Quixote fought windmills thinking they were real enemies. Similarly, medical device companies are finding themselves so focused upon satisfying current regulatory requirements that they are not developing sustainable strategies for the tsunami of regulatory complexity headed their way. The most significant drivers for the implementation of an enterprise labeling software system to date have been 21 CFR and the U.S. FDA's UDI. Future drivers include an onslaught of UDI variants that are coming from other regulatory bodies outside of the United States as well as pharmaceutical serialization in the Drug Supply Chain Security Act (DSCSA). The key to complying with these regulatory challenges is masterful control of master data. Adaptive master data management is key to effective labeling and regulatory compliance.
Qmed: How can enterprise labeling software systems help with today's quality assurance management challenges?
Batmanghelidj: An ELS has the potential of playing an incredibly significant role in quality assurance management. Aside from quality assurance for the labeling process through the automation of manual SOPs, an ELS can serve as the hub for a quality system by consolidating data and data quality assurance. Labeling, regulatory, and virtually any other form of data can come together in an automated system of checks and balances to ensure several vital synchronizations. These include ensuring that only the most recent version of an approved template is used, that versions of IFUs or eIFUs match appropriate versions of items, and more. At the high end of the scale of automated quality assurance, 100% print inspection ensures that the right data is on the right labels for the right items. At the mid range of the scale, labeling and regulatory data submissions can be synchronized automatically though a shared database. Other significant opportunities for synchronized quality assurance include making sure that label template redline markups match template changes that are being made.
Qmed: How can enterprise labeling software help medical device companies address supply chain concerns?
Batmanghelidj: Supply-chain concerns can be resolved through accurate and complete communications of vetted data. There are two primary touch points for the interactions that medical device companies have with their supply chain--data in and data out. Production supply-chain partners must have controlled access to maintain the data that they are responsible for with a level of automated and task-dependent project management. Production-dependent variable information from manufacturing must also flow in an accurate and automated manner. On the "data out" side, the system needs to be flexible enough to be able to communicate via established data pools or through custom communications protocols that supply-chain partners sometimes will require. Secured data within the medical device company then can become a definitive store for vital information needed by manufacturers and supply-chain partners alike. Anti-counterfeiting controls, just in time manufacturing, and powerful track and trace for recall all can now finally become a reality.
Qmed: Why is data security a concern today for medical device companies? Does everyone need to take steps to ensure security?
Batmanghelidj: For as long as data remains digital, data security will continue to be a hot topic in the news. Threats caused by malware and ransomware are presently taking center stage. The newly recognized potential of malicious code wreaking havoc on the health and safety of patients through compromised pacemakers, monitoring machines, and other critical, software dependent devices is onerous. Aside from traditional data security concerns about data at rest, data in transit, and user access security, the difficulty is in maintaining data security throughout the supply chain. This is where an enterprise labeling system with a life sciences-specific master data management system and native integration to serialization engines comes sharply into play. Everyone still needs to take steps to ensure security, but an enterprise labeling system that automates most activity with controlled access to reduce risks can simplify security immensely.
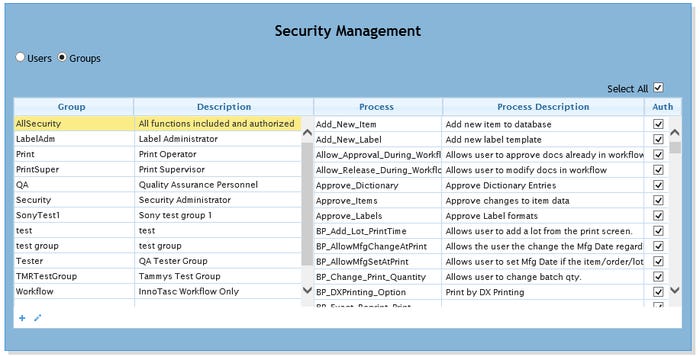
|
An example of labeling system security management. |
Qmed: What role does security play in enterprise labeling software?
Batmanghelidj: To minimize risk, security within enterprise labeling systems for the life sciences must be multifaceted. Traditional areas for software security consideration include protection for data at rest, data in transit, and user access security. But enterprise labeling systems can take things much further. Due to an opportunity to automate user access and permissions for 21 CFR compliance, enterprise labeling software includes a granular capability to grant and revoke software functionality by users or groups. Enterprise labeling software can control which plants, production lines, users, workstations, printers, etc. can print. It can control which label types, (pouch, carton, case, destination labels, etc.) can be printed and which label templates can be used. The potential to control and automate risky manual SOPs thereby reducing security risks is substantial.
Qmed: How is the changing state of data management, with increasing cloud storage options, influencing the utilization of enterprise labeling software?
Batmanghelidj: For the most part, data management with the advent of increasing cloud storage options is influencing the use of most enterprise labeling software systems in the same way. Separate integrations are being made between various cloud storage systems and enterprise labeling systems to deliver data. Most often, these integrations are separate and disconnected endeavors. While this may work, what is revolutionary in the industry is the concept of integrating a life sciences-specific adaptive master data management system.
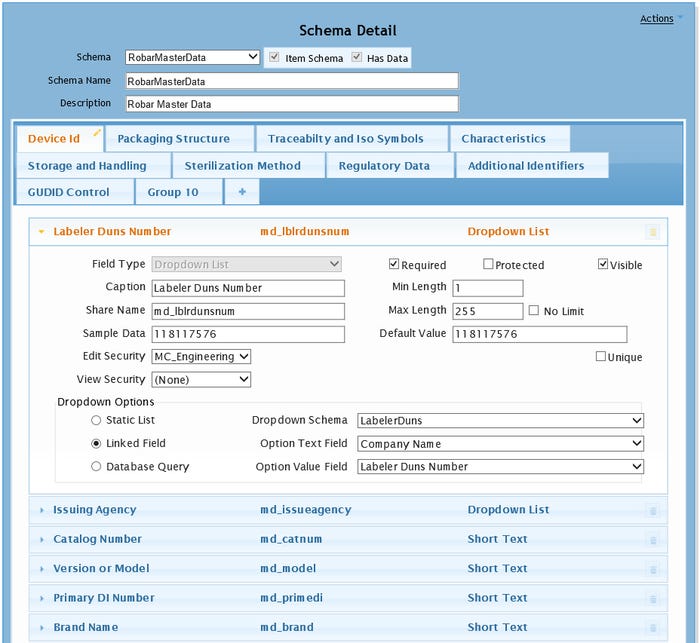
|
Adapting a database through configuration, not programming. |
Such a system adds a necessary level of flexibility and robustness to cloud integration. For instance, 21 CFR compliance capability can be built into a system that is validated for its capability to allow fields or schemas containing fields to be added on the fly. The system therefore doesn't need to be revalidated when changes are made. An auditable history for the changes in stored data values is automatically captured, and this affords a snapshot of where other systems were when their data was added or changed. When refreshing the data from entities such as contract manufacturers, this audit capability becomes indispensable. The system not only can serve as a pass through or as a repository for data that does not have a home elsewhere, it can also perform some surprising functions. Data values from different systems can be combined to create unique values and/or data transformation can take place. One of the most vital capabilities of a life sciences specific adaptive MDM is the automatic conversion to XML and SPL, the requisite data formats for data submissions in the life sciences.
Qmed: How do companies determine whether they use a cloud, a hosted service, or utilize on-premise data storage?
Batmanghelidj: Companies determine whether they use a cloud, hosted service or on-premise data storage for their data based upon the sensitivity of the data, their risk appetite, and their internal financial models. Most life sciences companies entertain the thought of storing less sensitive data in a multi-tenant environment in a pure cloud architecture. However, awareness of issues such as the comingling of data in a multi-tenant environment often complicates these decisions. Single tenancy in a hosted service offers a much safer solution, yet still provides the advantages of reduced IT overhead and the ability to fund labeling system costs as an operational expense. On-premise data storage is viable for companies that have available IT infrastructure and like the idea of making one-time licensing of an enterprise labeling system a capital expense. To understand the best option for a given company, it is necessary to delve more deeply into each of the data storage options for life sciences specific data.
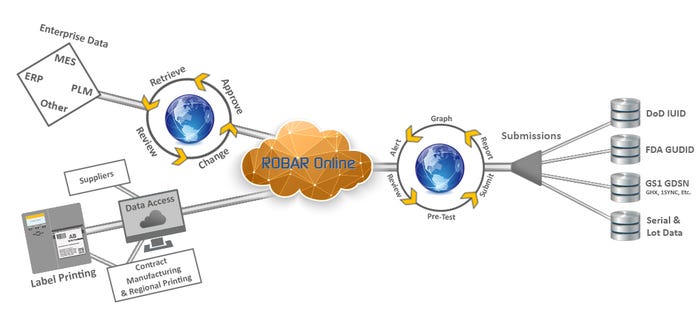
|
A cloud-hosted Life Sciences labeling system. |
Innovatum recently launched an online interoperable suite of solutions for enterprise labeling products, label design, management and printing, master data management, and communications technologies under the ROBAR product family.
[Images courtesy of Innovatum]
To learn more about all the health data being generated, attend BIOMEDevice Boston 2017 May 3-4 and hear these conferences and more:
A Doctor's Prescription for the Future of Connected Health Technology (Speaker: Joseph C. Kvedar, MD; Partners HealthCare)
Journey into the Future: The Road ahead for Medtech Wearables (Speaker: Mike Maczuzak; Smartshape)
Embedded Systems Safety & Security: Dangerous Flaws in Safety-Critical Device Design (Speaker: Michael Barr; Barr Group)
The Continued Rise of Wearable Technology Using Novel Biosensors (Speaker: Milan Raj; MC10)
A Connected Health Device Payers Love (Speaker: Hareesh Ganesan; TowerView Health)
Top Trends Impacting Medtech and IOT in 2017 (Speaker: Kevin Young; Continuum Innovation)
About the Author(s)
You May Also Like