6 Questions to Ask Yourself about ISO 11607 Compliance
These FDA-consensus standards guide packaging design and validation—how compliant are you, and how ready are you for the upcoming revision?
August 24, 2018
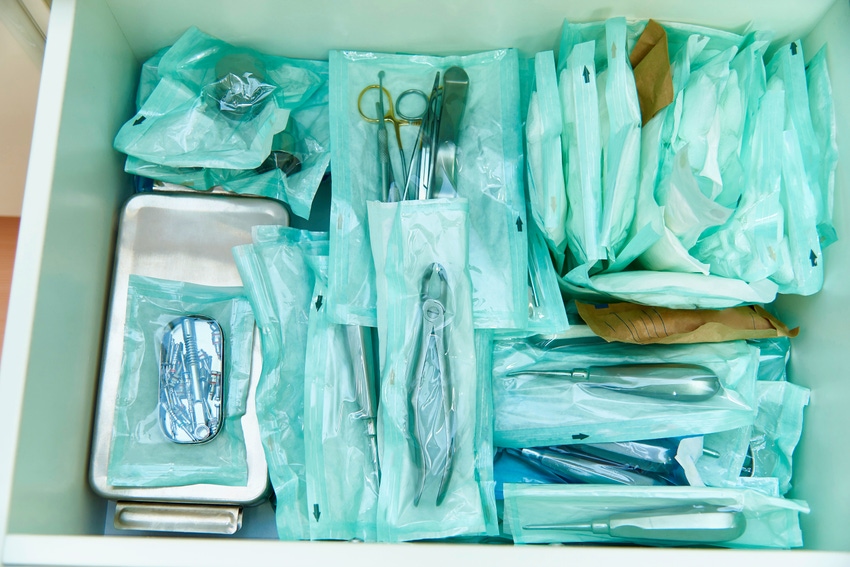
When working in medical device packaging, you should know that ISO 11607-1 and -2 are the recognized guidelines for validating terminally sterilized medical device packaging. The ISO 11607 standards are FDA-recognized consensus standards and European normative standards for CE marking. The ISO 11607 standards are in two parts: ISO 11607-1: Packaging for terminally sterilized medical devices — Part 1: Requirements for materials, sterile barrier systems and packaging systems addressing packaging materials and design, and ISO 11607-2: Packaging for terminally sterilized medical devices — Part 2: Validation requirements for forming, sealing and assembly processes addressing packaging process validations. The primary purposes of these standards are to ensure that medical device packaging allows sterilization, provides physical protection, and maintains sterility to the point of use. These standards are revised and updated every few years and are expected to be amended in late 2018 or early 2019.
Compliance with ISO 11607 is key to safe and successful medical device packaging, so how can your organization make sure your packaged devices are ready? Ask yourself the following questions for more confidence in your packaging system when you ship your devices or when your company faces an impending audit.
Does your medical device packaging system allow sterilization?
Your packaging must provide physical protection and maintain sterility up to the point of use for ISO 11607 compliance. Terminal sterilization refers to sterilizing a device packaged in a sealed barrier system. (Validating product sterilization is a separate process.) The device inside the package is sterile and remains sterile until the packaging fails or is opened. The minimum packaging required to keep an enclosed device sterile is called a “sterile barrier system (SBS).” All packaging around the SBS is called “protective packaging” by the ISO 11607 standards.
There are various sterilization methods, each with pros and cons for packaging materials. Referencing information from AAMI TIR 17 (Association for the Advancement of Medical Instrumentation (AAMI) Technical Information Report TIR17: Compatibility of materials subject to sterilization) or other resources pertaining to SBS material compatibility with particular sterilization methods is critical. Not all packaging materials are suitable for all types of sterilization processes; packaging materials could warp, melt, discolor, become brittle, or exhibit other compatibility problems. Even labeling materials could bleed, smudge, fade, fall off, or change in some way that impacts legibility when the sterilization process is not properly considered.
Protective packaging (often referred to in the United States as secondary and tertiary packaging) material choices also need consideration, as they, too, can be affected by the sterilization method. You do not want a case manufacturer joint failing because the adhesive used could not withstand an ethylene oxide (EO) sterilization temperature or the adhesive becomes brittle with radiation sterilization.
Device sterilization requirements need to match packaging material requirements, package performance, and shelf life expectations. Closely working with your company’s sterilization experts will ensure your packaging system is designed to assist in optimizing the sterilization process.
Does your packaging validation comply with the 115 “shall” statements?
There are more than 115 “shall” statements in the ISO 11607 standards. These shall statements must be met for your packaging to be ISO 11607 compliant. Some packaging aspects that are analyzed for the shall statements include packaging material traceability, validated test methods, sterilization compatibility, labeling compatibility, aging effects on packaging materials, distribution effects on the packaged product, and validated manufacturing processes.
Compliance with such “shall” statements takes a concerted effort as well as collaboration beyond the package engineering department, and it must be documented. Since aspects of both packaging design and packaging processes are included, working with departments such as quality, manufacturing, process engineering, logistics, marketing, sales, and end-users helps ensure all packaging is meets applicable shall statements.
Will you be ready for revisions to requirements that could be issued as soon as 2018?
ISO standards are reviewed at least every five years for potential changes. The ISO 11607 standards were written and published in 2006, with clarification updates in 2014. Standards are normally amended or revised every three to five years; the ISO 11607 standards are expected to be rolled out soon. The new revisions have been reviewed and are being processed for a formal vote this year (FDIS stage 50.00, in ISO speak). This means the final vote is this year with an expected release date at the end of 2018 or early 2019. To guarantee your organization’s readiness for the revised standards, it is best to assign someone to study the changes. This champion can then communicate the changes to the responsible parties in your organization. The biggest change currently expected is the additional requirement for documented human factors testing with packaging; this goes beyond the sterile presentation requirement currently in the document.
Do you know when FDA needs updated information about your medical device packaging?
Any changes to your existing device packaging require review by the regulatory affairs group (RA or it could be another acronym) within your organization. They will assess the impact to previous FDA submissions and approvals. Some potential packaging impacts could result in re-submission efforts, so those packaging changes could be delayed to align with other planned device changes or even be put on hold indefinitely. That is one of the biggest reasons to develop the “right” (optimized) packaging solution during initial development.
Would a checklist help?
To ensure your medical device product packaging is ISO 11607 compliant, your organization should have a thorough checklist aligning with its requirements. This checklist will help with audit readiness and could optimize your packaging during design and development. The process of compliance is multi-pronged and complex, with nuanced details than can change depending on each product’s specific purpose and circumstance. Although ISO 11607 compliance can seem like a daunting process, with attention to detail, inter-departmental cooperation, and experts dedicated to staying current on requirements and changes, your organization can be ready to send top-of-the-line packaged products to your consumers.
Are you ready?
About the Author(s)
You May Also Like


