Welcome to the Orthopedic Device Jungle
February 19, 2015
Whether it be the terra incognita of regulatory matters or the challenge of managing outsourced machining operations, orthopedic device makers might need a machete to cut through the thicket of uncertainty in the industry.
Brian Buntz
Making orthopedic devices is anything but straightforward these days.
|
A technician performs a limulus amebocyte lysate (LAL) test, which may soon be required for orthopedic implants. |
While most orthopedic devices are made of common metals, plastics, or ceramics, it can be difficult to establish their long-term biocompatibility because it is not feasible to do long-term trials to gauge how they react in the human body over time. "There is no such thing as a 20-year animal study before an orthopedic device is approved for human use," John S. Bolinder, vice president of marketing and communications at Nelson Laboratories (Salt Lake City, UT) at MD&M West.. "We are learning as we go with orthopedics in clinical use."
One of the most prominent examples of this learn-as-we-go dynamic is the metal-on-metal hip implant, which, when it debuted, was vaunted as having improved wear resistance over prior designs. Initially cleared by FDA via the 510(k) pathway and via the CE Mark system in Europe, metal-on-metal hip implants were thought to be substantially equivalent to hip implants already on the market. But several recalls and billions of dollars worth of lawsuit settlements later, metal-on-metal implants have emerged as a kind of case study in what can go wrong when orthopedic companies test new product ideas. In this case, as many as half of such hip implants had to be replaced after six years due to metallosis and other complications, according to The Guardian newspaper in the United Kingdom. Similar issues with osteolysis have occurred with ultra high molecular weight polyethylene for total knee arthroplasty.
As a result of the metal-on-metal hip implant fiasco, the regulatory bodies have become more risk-averse with regards to orthopedic implants in general and are continuing to rethink their approach to deeming products to be safe.
Another challenge for the sector is the precision required to produce orthopedic components. Many parts are machined from hard metals such as titanium or stainless steel--operations that require a very specialized skillset to do well. As a result, many orthopedic companies rely heavily on third-party manufacturers to perform such operations, and have little to do with the actual manufacture of their own product. Oftentimes, the original equipment manufacturer (OEM) ends up being something like a contract packager, putting together the various pieces for surgical kits and implants used in orthopedic procedures during the final step of manufacturing. In cases like this, it can be difficult for OEMs to maintain solid process controls over their production process when a considerable amount of it is performed outside their own facilities.
The result is that regulatory agencies like the FDA and the British Standards Institution (BSI) have been coming down on orthopedic device makers for poorly managing their contract manufacturing operations, and are frequently deciding to conduct audits of them.
It all means that orthopedic device companies have plenty of challenges to face in the new regulatory environment. And although a device many be described as "substantially equivalent" the manufacture of the device may vary. Here are several examples that are especially worth noting:
Messy Machining
The machining and milling that are widely employed to produce orthopedic implants are messy operations. In the process, shavings from the metal can fly off, and after the procedure is over, the product is still coated with an aqueous or oil-based residue that were used as cutting fluids in the machining process. Other contaminants includes microbiological and particulate debris. Failing to get those manufacturing residues and contaminants off can cause big problems for patients.
Also worrisome is the particulate debris itself caused by the machining process. In some machining facilities, metallic particle debris is visible in the air from machining, polishing and finish processing. In some cases manufacturers fail to use an air wash or water wash to clean these products after deburring, and put them directly into the final packaging.. "Sometimes, you can actually see metallic flakes in the packaging," Bolinder says.
For patients receiving implants with metallic particle debris on them major problems can arise. "For instance, I read an article about eight years ago, about a patient with a metal knee implant who died from a stroke two weeks later," Bolinder says. "Why? Several complications including infection and particulates coming off the device, entering the cardiovascular system and ultimately causing his heart to stop." It is imperative to know the cleanliness of the devices in their finished state. Anything on the device, but not intended to be part of the device, is a problem.
Patients receiving an implant with metallic debris face a similar fate as those who received metal-on-metal implants. Both create contamination once they go into the body.
"Any time you get particulates in the bloodstream, you have a problem," Bolinder says. Metal shavings are problematic because metal doesn't metabolize. "Once it gets into the body, it doesn't get out. So a patient starts building up a toxicity level, developing systemic toxicity issues or experiencing device failure and discomfort when the metal contributes to wearing within a joint and adhesion issues with tissue at the implant site."
|
Examples of residual materials commonly found on medical devices following manufacture that may induce unwanted toxicological risk. |
Water System Risks
While using a water system to clean machined orthopedic products can help protect patients, there has also been a recent uptick in FDA citations related to orthopedic firm's use of such systems as water can be a source of endotoxin.
In addition, it is difficult to maintain strict change control over a firm that is machining orthopedic parts. "A machine shop working on orthopedic parts is kind of like a machine shop making auto parts--they are working on engines, rods, and various other things. Their volumes are ebbing and flowing and their product range is constantly changing," Bolinder says. "But they are using the same water bath, the same processes, and the contamination is changing." In either case, machine shops likely don't have dedicated pieces of equipment for certain types of parts, which would help them better keep track of potential contamination, whether it be a cutting fluid or a detergent used in the process. "You really have to assess all of the process changes that occur as their manufacturing changes day to day," Bolinder says. "Can you really do a one-time end-point validation test to make sure the product is safe when the manufacturing process is constantly changing? Manufacturers need to understand and control their processes, or those of their third party partners, to ensure additional contaminants are not introduced due to process variability."
Moving Regulatory Goalposts
Another challenge is that orthopedic firms must have a solid understanding of FDA's changing expectations, some of which are now ambiguous. Even seemingly basic criteria, like the cleanliness of medical device components, are undergoing FDA review.
"One thing we are facing right now has to do with the uncertainty around a guidance document the FDA released nearly two years ago in April 2013," says Thor Rollins, biocompatibility specialist at Nelson Laboratories.
Titled "Use of International Standard ISO 10993, 'Biological Evaluation of 2 Medical Devices Part 1: Evaluation 3 and Testing,'" the document was the first guidance on the subject since 1995. "This new draft guidance had a lot of things that FDA were enforcing but hadn't released guidance around it," Rollins says. "There were a couple of things that scared us though. Two of them specifically had the potential to impact orthopedic companies."
Detecting Toxicity
The first matter of concern to orthopedic device companies relates to a sentence in the FDA guidance document that has to do with endotoxin testing. "The endotoxin is a specific pyrogen from gram-negative bacteria. It is found in their cell walls and is not removed from products with sterilization," Rollins says. "It is a critical element of cleanliness to remove pyrogen from medical devices, especially ones that have vascular system, ocular, neurological or cerebral spinal fluid contact.. Many medical device companies in cardiovascular, drug delivery and neurological fields have always tested for it. For many orthopedic companies this is new," Rollins explains. In the past, FDA's thinking was that a device had to be in the vascular system to be covered by this rule.
The latest draft guidance though, instead of saying vascular contact, cerebral fluid, and all of those normal contacts, states:
Implants, as well as sterile devices in contact directly or indirectly with the cardiovascular system, the lymphatic system, or cerebrospinal fluid (CSF) (regardless of duration of contact), and devices labeled as "non-pyrogenic" should meet pyrogen limit specifications.
Most orthopedics companies don't do this test right now. "The first comma in that sentence suggests that all implants that directly or indirectly contact the patient in this manner must conform to device guidance for bacterial endotoxins and the limulus amebocyte lysate (LAL), test," Rollins explains.
The potential impact of this draft guidance, if it means that orthopedic companies must do this test, is that they would be required to do it for every lot. "We are checking with the FDA to make sure that is their intention. But we still haven't heard back," Rollins says, adding that the agency might weigh in on the matter in the months to come.
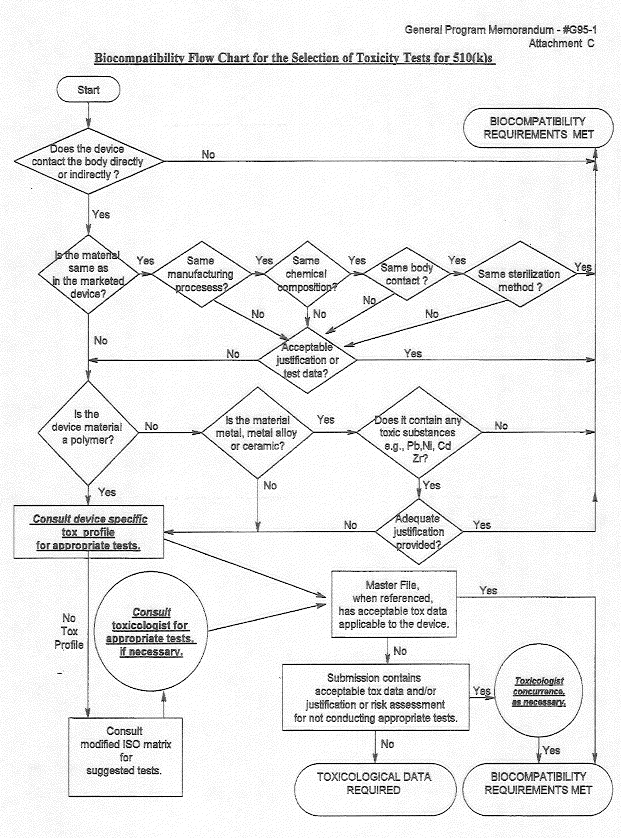
|
Draft guidance released by FDA in 2014 could eventually replace the agency's Blue Book Memorandum #G95-1 on biocompatibility. |
Questions Related to FDA's G95
The other thing that could be of concern for orthopedic device makers is a chart that has long been a part of FDA's G95-1 memorandum, which relates to the ISO 10993 standard. The idea of G95 is that you look at the contact type of your device and the duration in a standardized matrix, and it tells you what kind of biocompatibility test to conduct for the medical device. It helps you plan based on which considerations have to be used for that device to be tested," Rollins says.
There were a number of test categories pertaining to this that FDA considered optional. "That means if I am an FDA reviewer and I am not familiar with your material, or I am suspicious or concerned in any way, I can require those types of tests beyond the guidance matrix," Rollins says.
But the most recent relevant draft guidance would not view such categories as optional, but require that device firms justify why they are not doing them through a formal, documented risk assessment. "For instance, if I have a device with limited contact to circulating blood, genotoxicity testing has been traditionally optional. But now we see FDA asking manufacturers to either justify why they didn't do those tests or to do them," Rollins says.
Another good example from orthopedics is a delivery device for a screw or a surgical tool. "It could be an external communicating device used for less than 24 hours that could have an optional pyrogen test for toxicity," Rollins says. Traditionally, orthopedic device firms haven't used this pyrogen test for such parts, which are often made of materials like PEEK, stainless steel or titanium. But as a result of the new guidance, device firms would either have to do them or justify why they are not doing them.
On the positive side, FDA is putting a greater emphasis on risk assessments for materials based on scientific principles. A couple of years ago, the FDA would view the risk assessment process as a sort of checklist of tests. "But now, in the latest draft document, it says that it is not a checklist, but these are the categories you have to consider, but you don't necessarily have to test for them," Rollins says. "You could address those through evaluation of literature, chemical and material characterization, and other means to evaluate risk and address it other than just biocompatibility testing."
This is good news for many orthopedic firms since most of their products are made from well-known materials like titanium or stainless steel using standard manufacturing procedures. Orthopedic firms are often able to justify out of testing based on that and a documented understanding of their device cleanliness.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like