Using CFD to Gain Insight into Medical Device Designs
Computational fluid dynamics (CFD) is a branch of fluid mechanics that uses numerical methods to solve and analyze problems that involve fluid flows. Flow modeling with CFD software lets you predict and visualize physical phenomena occurring within or around your medical devices. Traditionally used in the aerospace and automotive industries, CFD is gaining rapid acceptance and use for medical, pharmaceutical, and biomedical applications. Key focus areas include guiding new product development, improving manufacturing processes, and predicting device or drug performance in situ.
May 17, 2012
A few examples where CFD has played an important role include:
Optimize the exterior shape of intravascular devices to improve their performance and minimize their disturbance on the surrounding blood flow field.
Identify regions of flow recirculation, remove unneeded flow volume, and hence reduce the potential for blood clot formation within your device.
Improve the coating uniformity on devices such as cardiovascular stents by modeling and refining the spraying technique.
Predict the distribution and depth of deposition of inhaled drug particles in the human lungs using CT-scanned images of the lungs.
Assess the change in drug concentration with time for an implanted drug-coated device based on the local tissue properties and adjacent fluid environment.
CFD is also gaining recognition as a valuable tool for supplementing test data needed for regulatory submission and approval. An FDA/NHLBI/NSF-hosted workshop on computer methods for medical devices held in 2011 is one example of the effort underway to fully leverage the benefits of numerical simulation. This conference presented advances in both solid and fluid mechanics modeling of cardiovascular and orthopedic devices and the associated validation results. Future conferences are aimed at expanding both the physics studied, such as including electromagnetics, and the fields-of-use. The insight gained from using CFD, when combined and validated with experimental data, provides a strong technical foundation upon which sound design decisions can be based. Focused CFD efforts enable rapid consideration of design alternatives, explore performance across a range of operating parameters, and ultimately help reduce the overall development time and cost in bringing a new technology to the medical marketplace.
CFD provides both detailed flow-related information and overall performance assessments that can be used to guide the direction of product development. |
CFD provides both detailed flow-related information and overall performance assessments that can be used to guide the direction of product development. Such numerical design iterations can often reduce the number and cost of expensive and time-consuming prototype fabrication and testing. Using CFD analysis, manufacturing processes that involve fluid flow can be optimized from both a quality and cost standpoint. For instance, if your process involves the controlled curing of a polymer coating to promote good adhesion with the substrate, one can model the impact of local air temperature and flow conditions on the rate and uniformity of the curing process. The technology is also promising for simulating human physiological flows that interact with devices or affect drug-delivery performance; obtaining detailed measurements for such flows is often difficult, time-consuming, and expensive to measure using animal or other physically-representative models.
Many biomedical applications involve fluid flow and heat/mass transport in a device or within the human body. Some examples include blood pumps, artificial heart valves, blood oxygenators, filtration devices, catheters, tubing, aerosol drug delivery, and diagnostic equipment. Such CFD models can include the effects of magnetic fields, gas transport, multi-phase flow, deposition of particles, deformation of solid regions surrounding the fluid, and biochemical reactions. CFD analysis offers details of fluid velocities, pressures, solute or particle concentrations, temperatures, fluid stresses, and heat/mass fluxes throughout your entire device.
These computed flow-related parameters can be displayed in different formats—including color-coded images—revealing the fluid-related inner workings of your device. CFD is an excellent tool for conveying the functionality of your new device to others, including your management and development team, future customers, and regulatory agencies. As part of an analysis, engineers can alter model geometry, boundary conditions, or material properties to determine the effects on the system under study. As a result, CFD is well suited for conducting parametric studies, making it possible to evaluate more design alternatives than with traditional build-and-test methods, thereby allowing for faster performance optimization and significant reduction of design cycle time.
While experimentation using physical and animal models is needed to demonstrate the actual performance of a new device, it has some important limitations that explain the increasing emphasis many device manufacturers are placing on computer simulation. Experiments take a long period of time to perform, are expensive, and, in certain cases, may involve risks to animal or human subjects. For these reasons, device manufacturers are turning to computer simulation to evaluate the relative performance of various design alternatives over the full range of intended use in an effort to further ensure the safety and effectiveness of their products.
Another problem with physical testing concerns the limited quantity or accessibility of the data, which are obtained only at those locations where measurements can be made. Computer simulations, by contrast, can provide calculations of as many relevant parameters, and in as many locations, as the analyst requires. CFD models provide detailed flow information and insight even in regions where physical measurements cannot be made.
CFD benefits include the abilty to:
Analyze. You can graphically visualize any number of cause/effect relationships to improve device performance and effectiveness. With a CFD model, you can easily change and assess the impact of different fluid properties or composition as well as the boundary conditions (i.e. flow rate, pressure and temperature) on your device’s performance. This is particularly useful when evaluating your device over its intended range of use.
Optimize. You can quickly evaluate design options prior to laboratory testing to save time and money while demonstrating product viability and performance. With detailed flow-related information, such as local speed, pressure, and temperature conditions, one can make design refinements that can be critical in balancing design trade-offs and achieving the intended performance goals. In a blood oxygenator, for example, gas transfer increases as the blood shear rate adjacent the gas transfer surface increases. However, it is critical to stay below shear rates that could cause blood cell damage or lead to platelet activation. Using CFD, both the surface blood shear rates and gas transfer can be predicted and the flow path geometry adjusted to optimize the overall device performance.
Understand. You are able to evaluate implications of changes in design or process variables to rapidly gain a wider knowledge base. Having the ability to see the flow patterns within your device over a broad range of operating conditions gives you the ability to determine the direction for your design changes and fully advance your technology.
Troubleshoot. You can understand and diagnose problems quickly prior to confirmatory experimental testing. If unforeseen performance issues occur when moving from bench testing to in vivo evaluations, such as platelet deposition, CFD analyses can prove invaluable in helping to uncover the source and guide the direction for resolving the issues. The CFD simulations can identify both high and low shear stress regions where platelets could be activated and deposited. Geometry changes can then be made to eliminate such regions and the resulting performance confirmed through new in vivo tests.
Reduce time-to-market. You can speed product development by modeling multiple scenarios, thereby ruling out unsuccessful attempts before conducting time-consuming laboratory tests—ultimately compressing your development cycle. Key here is the efficient use of your company’s resources. The goal is to bring safe, high quality, and well-designed devices to market quickly. Fully leveraging the capabilities of CFD to direct the development path and support the experimental testing is a prudent means to achieving this goal.
Conclusion
In summary, CFD offers your engineering teams the ability to visualize and better understand the key design trade-offs affecting the performance of you new design or manufacturing process. With this knowledge, they can select a preferred design and then navigate through bench and in vivo testing that is needed to validate the modeling methods and confirm the device’s performance. The ultimate goal is to efficiently deliver a new product that has a solid technical foundation and, as a result, will function reliably and effectively when used clinically.
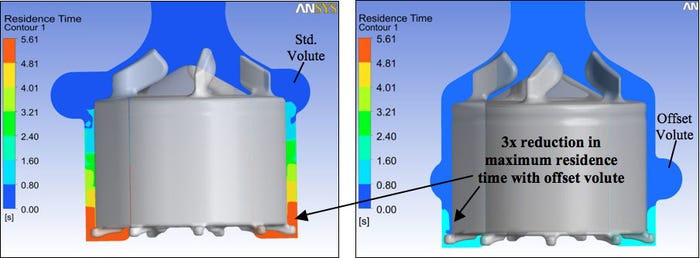
Case Study: Cleveland Clinic Right Ventricular Assist Device (RVAD). One of the keys in successfully applying CFD is to clearly define your analysis objectives. For example, the research team at the Cleveland Clinic was developing a new centrifugal blood pump for right ventricular support (Figure 1). Key to the success of this new pump was achieving the desired hydraulic performance (i.e. pressure increase), limiting the peak wall shear stress to minimize the potential for blood cell damage, and providing uniform wash-out to reduce the likelihood of blood clot formation in re-circulating flow regions. Early in the program, CFD studies of the pump incorporating a standard volute design, one that is in-line with the pump’s primary impellers, showed good values for hydraulic performance and wall shear stress levels. However, the time the blood resided within the pump (i.e. blood residence time) was significantly increased in the lower regions of the pump.
|
Figure 1. Color contours of velocity in stationary frame on pump rotor. On the left is the standard volute design, while the image on the right depicts the offset (lowered) volute design. |
To address this flow non-uniformity, the Cleveland Clinic team proposed a lower, offset volute design. The resulting CFD analyses for the new volute design provided a significant increase in flow between the inner rotor and the outer pump housing as the blood flowed from the primary impellers to the volute. This enhanced washout of the lower rotor surfaces yielded a 3× improvement in the overall uniformity in the pump’s blood residence time (Figure 2). This significant reduction in average residence time provides a larger margin of safety related to the pump’s potential for blood clot formation. With the offset volute design, hydraulic performance and wall shear stress values were still within the desired range. In vitro experimental studies, via hydraulic performance testing and dye wash-out studies, supported the CFD predicted pump results.
|
Figure 2. Color contours of residence time, centerline plane cut. As was the case with figure 1, the image on the left shows the standard volute design, while the image on the right shows the offset (lowered) volute design. |
In summary, CFD simulation was used to uncover and highlight regions for pump design improvements. It was then used to assess the impact of design changes that were confirmed experimentally. The CFD analyses revealed flow details throughout the pump that were difficult to observe experimentally and helped verify the benefit of the new offset volute design.
Note: SimuTech group used both ANSYS-Fluent and ANSYS-CFX to perform the CFD analyses in support of the Cleveland Clinic’s right ventricular assist device development.
Mark Goodin is an experienced CFD consulting engineer at SimuTech Group (Hudson, OH). He specializes in CFD simulation of medical devices with particular expertise in cardiovascular device simulation and product development. He holds a master's degree in engineering from Massachusetts Institute of Technology and a bachelor's in engineering from University of Illinois at Urbana-Champaign.
About the Author(s)
You May Also Like