Document and Change Control in the Design Process
Medical Device & Diagnostic Industry MagazineMDDI Article IndexOriginally Published April 2000When properly implemented, document and change controls reduce product defects, minimize field problems, and increase end-user satisfaction.
April 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Originally Published April 2000
When properly implemented, document and change controls reduce product defects, minimize field problems, and increase end-user satisfaction.
Document control starts early in the design process and extends beyond the initial release of a design through the life of a product. Design-output documents form the basis for the device master record (DMR) that is ultimately transferred to production. Document and change control help bridge the gap between production and design control.
Discipline and communication are the keys to effective document and change control systems. Knowing how to make crucial decisions regarding design and production changes and understanding what controls to apply for each are vital skills for manufacturers. This article reviews the regulatory requirements and discusses how to implement systems for document control. It covers the basics of establishing and maintaining systems for controlling document review, approval, and distribution, and explains how to integrate ongoing design change control with systems for document and change control.
REGULATORY REQUIREMENTS
Document control is an essential element of an effective quality system. Documents demonstrate that the systems and controls used to ensure that finished devices meet specification have been effectively implemented. They communicate desired outcomes, clarify methods, and ensure consistent results. When implemented properly, document controls help reduce the likelihood of defects and minimize field problems. Ultimately, these improvements increase customer satisfaction.
The requirements for document controls are defined in section 820.40, subpart D, of the quality system regulation (QSR) for medical devices.1 The general requirements are to "establish and maintain procedures to control all documents required by this part." In this case, "all" can be broken down into two main categories: quality systems procedures and DMR documents (see Table I). During design and development, the documents of interest comprise a substantial portion of the design history file, which will eventually be transferred into the DMR. It includes both hard-copy and electronic formats.
Quality Systems Procedures | Device Master Record/Design History File |
|---|---|
Manuals | Specifications and drawings |
SOPs, work instructions | Process equipment and controls |
Forms | Inspection and test procedures |
Approved vendor lists | Acceptance criteria |
Purchase orders | Packaging and labeling |
Installation, maintenance, and service procedures | |
Table I. Examples of documents to control. |
All documents must be reviewed for adequacy and approved prior to distribution. The approvals should be documented and the controls must ensure that the right documents are used at the right location. In many cases, the right document is the most current revision or issue. This means that old or obsolete revisions must be removed from points of use. If down (obsolete) revisions must be maintained to build spares, then controls should be established to prevent the accidental use of the wrong document, and to ensure that the correct revision is in use.
FDA recognizes electronic signatures on documents if appropriate controls have been established to ensure that each electronic mark or identification can only be issued by the individual authorized to use the mark and approve the document.2 Electronic documents should be controlled in a manner similar to hard-copy document control, which includes protection against unauthorized changes.
When changes to documents occur, the changes must be reviewed and approved using the same methods used initially to review the documents, unless another method can be justified. This helps ensure that a document will have the same level of scrutiny as it did originally. In addition, provisions should be established to communicate changes to appropriate personnel and to maintain a change record.
Several other sections of the QSR relate to document control as well. Both the sections for purchasing data (820.50(b)) and production and process changes (820.70(b)) reference document controls (820.40). In addition, design and document controls are intertwined in the regulation. Subpart C, design controls (section 820.30(h), design transfer), requires manufacturers to "ensure that the design basis for the device is correctly translated into production methods and procedures." Typically, the last step in this process is the review and approval of the DMR.
Finally, the section on design changes, 820.30(i), requires that design changes are identified, documented, validated (or verified, if appropriate), reviewed, and approved before implementation. It is typical for companies to have two systems: one system with simpler controls to manage the design configuration during design and development, and one to manage the quality systems procedures and DMR documents.3 It is possible, however, to use the same system during the entire design process.
GETTING CONTROL OF DOCUMENTS
The first question manufacturers often ask is when in the design process they should initiate document control. Even in start-up companies where manufacturers are developing their first product, a quality systems manual is needed. Once a decision is made to develop a product for market, detailed procedures for design control must be put into place. In addition, support systems such as management responsibility, training, purchasing control, calibration control, and, of course, document control, should be well defined. As soon as the design control process begins to generate design output, all documents must be controlled (see Figure 1). Furthermore, the output from any design stage must be reviewed and approved. This includes design input and output documents. If changes are made, the requirements for design change control apply. Keeping a record of corrections to design deficiencies is an important part of the design history file and helps prevent similar mistakes in future changes.4 Any design reviews, risk analyses, verifications, or validations should be updated or repeated to ensure that the design output meets the design input, and that device specifications consistently conform with user needs and intended uses.
|
Figure 1. When document controls start. |
Figure 1 illustrates that as the design is being transferred and translated into the DMR, documents must be reviewed and approved to ensure the design is correctly translated into procedures and specifications for manufacturing. This does not happen all at once, typically, but occurs throughout the development effort as design elements are completed.
Figure 2 gives some tips on things to consider when reviewing DMR document adequacy. The list is not exhaustive and each item may not apply to every document, but the checklist does provide a general starting point for document review. Document reviews can be done as part of the final approval or prior to final approval. If performing the review as part of the final approval by routing the document for signoff, manufacturers should be careful to get reapproval if changes are made during the signoff process. For this reason, many organizations have review and signoff meetings to collect all input during final review; there, changes are made as needed and the final approval is documented.
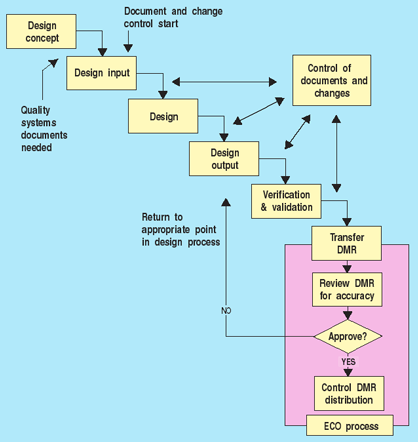
Figure 2. DMR document review checklist.
Manufacturers must decide which approval authorities will work best in their organization. It is important to monitor how the review and approval process is working, and to alter it when necessary. For example, if frequent change orders are required to correct items missed during the review and approval process, then the root causes should be identified and the necessary improvements made.
CHANGE CONTROL
Defining change is simple. Any alteration of an existing approved document is considered a change to the document. The forces driving change may include:
Quality problems from corrective and preventive actions.
Marketing or customer needs.
Regulatory or standard changes.
Productivity or cost improvements.
Vendor or raw material changes.
Document improvements or use of a different format.
There are two principal areas for change, the design and the process. A design change is an alteration of the device's design basis. The impetus behind design change is frequently field or other quality problems. Process changes alter process control methods, but are not intended to alter design; however, a design change may force changes to the process. When dealing with design changes, manufacturers need to decide what types of design controls to apply.
Figure 3 outlines a process for deciding what types of change controls are necessary. If the change is to the process only, production change controls can be used for implementation. If, on the other hand, the change involves the design, the first determination to be made should be if the device's intended use will be the same. If it is not, the manufacturer must start at the beginning of the design process. All steps need not be repeated in full, but each must at least be considered and determined to be unnecessary before moving on. The flowchart (Figure 3) may differ depending on the specific design process, but it does provide guidelines for making decisions. In addition, manufacturers also need to determine if the submission of a 510(k) premarket notification or a premarket approval supplement is required. Decisions made using the flowchart in Figure 3 can help support these submission determinations.
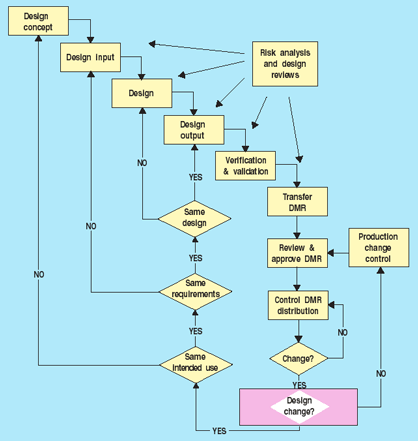
Figure 3. Change control decisions.
In addition, some sort of mechanism should be established to help facilitate making the change control decisions. A checklist, for example, can help filter out some of the more routine changes that occur on an ongoing basis—things like document corrections or improvements. Risk analysis can also be used to determine the appropriate level of design controls. Once manufacturers have decided where to start in the design process, they can apply appropriate design control procedures.
Simple low-risk design changes can be processed with appropriate verification, validation, and design review. Complex and higher-risk changes should be more thoroughly planned and more-extensive verification, validation, and design review should be performed. It is important not only to consider the verification and validation requirements for the proposed changes, but also to determine the possible adverse effects on other device functions. In either case, cross-functional team members should review these decisions to ensure that appropriate design controls are implemented.
Manufacturers also have to decide if the change warrants a revision to the existing document, or mandates the creation of a new document and part number (or document number). This decision requires careful consideration, as changes can impact other documents and may require costly alterations to the production planning system. The usual decision-making criteria involve evaluating whether the change alters form, fit, or function. If any one of these criteria changes, then a new part or document number may be in order.
Essentially, manufacturers are deciding if the old and the new are interchangeable. Sometimes this is a practical matter: if the old version didn't work well and is no longer needed, then changing the part number will help prevent errors. If the change has altered form, fit, or function, the manufacturer may also have to consider retrofit options for existing devices.
PRODUCTION CHANGE CONTROL
Oftentimes design changes are processed using production change control procedures. This is either because a process change is being made, or because the manufacturer wants to facilitate the transfer of the design change into production. Production change control procedures provide systems not only for guiding review and approval, but also for evaluating the change and integrating it into the normal flow of production. Factors to consider for a production change control system include:
The need for process validation.
Plant tests or pilot runs.
Disposition of test materials.
Change effectivity and traceability.
Disposal of obsolete inventory.
Communication and training.
These issues need to be addressed early on in the change process. Waiting until later in the process can delay approval, because the issues must be resolved to the satisfaction of the approvers. Sometimes a change-request system is established to plan and authorize the change up front. In this type of system a requested change and subsequent plan to control the implementation are proposed, reviewed, and approved. The plan may include appropriate design controls, process validation, and other implementation considerations. Once approved, the plan is implemented. If all criteria for the change have been met, the change is implemented using a change order.
The key to answering validation questions is to understand how the process has been changed. If the process is new, the main question is, can it be fully verified by subsequent inspection and tests? If not, it must be validated. If the process is modified and was not previously validated, then the same question applies. Previously validated processes require revalidation, or at least justification as to why the existing validation supports the changes. If deviations occur, however, manufacturers need to consider revalidation. For example, if changes are being made to a process because defects were too high—say yields were only 95% while the previous validation boasted of process yields of 99.9%—then the old validation was probably inadequate and revalidation is necessary. Using process validation tools can also help establish the effectiveness of process changes, as opposed to using the production environment as a proving grounds.
Process validation or other qualification studies of an existing process can be done using plant-test or pilot procedures. Since this is typically done in production and the changes have not been reviewed and approved, a temporary change notice is required to control prototype documents and any product produced. A product made during test runs should be quarantined until all testing is complete and the final reviews and approvals have been made. The general rule for final disposition of a product made during a test run is to ask, Has the final configuration of the documents changed after the test was run? If the answer is no, then once the change has been formally reviewed and approved, the product can be used. If the configuration has changed, the manufacturer has to decide how significant the changes were, and then be able to justify the release of any product produced. If justification cannot be made, revalidation is required.
When deciding at what point to cut a change into production, manufacturers must take into consideration the need for the change, the inventory of old material, and the availability of new material. If the old design is unacceptable because of product risk, the change should be initiated immediately and the old inventory scrapped or reworked. To do otherwise requires the manufacturer to balance the economics of reworking the old inventory with the benefits of cutting the change in and the availability of new parts.
Communication and training considerations are vital for changes to be successful. In many cases changes will need to be closely monitored to minimize the impact of problems on production. Operators often need to be retrained in order to implement the process changes.
CONCLUSION
Starting document controls early in the design and development life cycle helps prevent problems down the road. These controls apply to both quality systems procedures and DMR documents. Review and approval controls should help prevent the release of inaccurate or inappropriate documents. Distribution controls should be simple, visible, and ensure that only the right documents are used. Change controls should focus on planning, validating (or verifying), and implementing changes to minimize potential quality problems. The keys to effective document and change controls are:
A well-defined process.
Attention to detail.
Communication.
Discipline.
Validation or verification of changes before implementation.
REFERENCES
Code of Federal Regulations, 21 CFR 808, 812, and 820.
Code of Federal Regulations, 21 CFR Part 11.
Design Control Guidance for Medical Device Manufacturers, (Rockville, MD: Center for Devices and Radiological Health, FDA, March 11, 1997).
Trautman, K., FDA and Worldwide Quality Systems Requirements Guidebook for Medical Devices, ASQC Quality Press, (Milwaukee: 1997).
Andrew Snow is a quality assurance consultant with 20 years of engineering and management experience.
Return to the MDDI April table of contents | Return to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
You May Also Like


