CDRH Unveils Medical Device Innovation Pathway
Bombarded by demands that it be more supportive of medical product innovation (see, for example, the next story), FDA in February announced its Medical Device Innovation Pathway, including a priority review program for a few breakthrough devices.
March 1, 2011

|
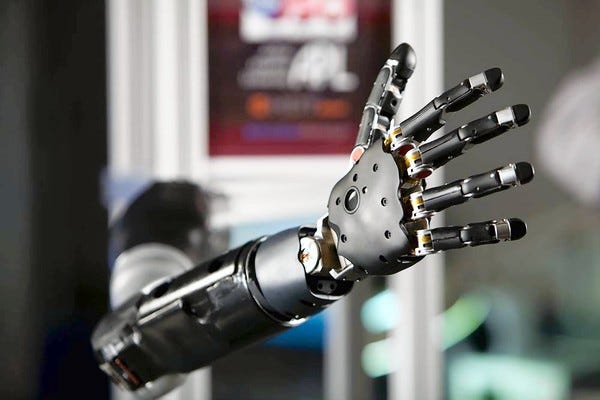
A brain-controlled prosthetic arm funded by the Defense Advanced Research Projects Agency will be the first device evaluated in FDA's new innovation pathway. Image courtesy of JOHNS HOPKINS UNIVERSITY APPLIED PHYSICS LABORATORY |
Bombarded by demands that it be more supportive of medical product innovation (see, for example, the next story), FDA in February announced its Medical Device Innovation Pathway, including a priority review program for a few breakthrough devices.
The review process will be piloted on a brain-controlled, upper-extremity prosthetic being developed by the Defense Advanced Research Projects Agency (in collaboration with Johns Hopkins University). The prosthetic was demonstrated during a Webcast announcing the initiative. It is intended to restore near-natural arm, hand, and finger function to patients suffering from spinal cord injury, stroke, or amputation. Officials said a test of the brain chip that would control the device could start within six months.
An agency news release said the innovation pathway program includes the following features:
Products would have to be truly pioneering technologies, with the potential of revolutionizing patient care or healthcare delivery.
Selected products would receive an innovation pathway memo from CDRH with a proposed roadmap and timetable for device development, clinical assessment, and regulatory review.
Products would be assigned a case manager, their important scientific issues would be identified and addressed earlier in the development process, and they might be able to qualify for flexible clinical trial protocols.
CDRH director Jeffrey Shuren told the Webcast audience that applications will be reviewed by the Center Science Council, a new oversight body being developed within CDRH. The council will have senior managers and experienced scientists who will facilitate the device development and evaluation process. He stressed that the new process and pathway does not involve any changes in regulatory requirements or standards for evaluating submissions. Rather, it involves CDRH becoming active much earlier in the process and providing greater clarity and predictability to sponsors.
Asked about resources needed for the innovation pathway, Shuren said that the agency’s current budget cannot support a radical change in approach. However, the agency does have the funds to take on one or two devices a year using the new approach.
A CDRH white paper on the innovation pathway says that there are several actions the center could take to help accelerate and reduce the cost of development and regulatory evaluation of innovative medical devices. Such actions include establishing the priority review program, streamlining the de novo pathway, establishing a voluntary third-party certification program for U.S. medical device test centers, and creating a publicly available core curriculum for medical device development and assessment. The white paper also mentions leveraging device experience and data collected outside the United States; advancing regulatory science for medical devices through prioritizing scientific research, establishing public-private partnerships, collaborating with other government agencies, and holding public workshops; and seeking public input to identify important and innovative medical device technologies as they arise.
AdvaMed issued a statement praising FDA for recognizing “the vital role and efficient and predictable regulatory system plays in ensuring American patients have timely access to the latest treatments and cures and in helping the U.S. maintain its world leadership in medical innovation.” The organization said it would discuss specifics of the new proposal with agency officials. It cautioned that FDA must maintain its focus on bringing greater speed and consistency to the current review process and on addressing the full spectrum of concerns that have contributed to recent problems. These issues include unacceptable delays and inconsistencies that AdvaMed says needlessly delay patient access to life-saving and life-enhancing technologies.
Senators Seek to Change FDA to Foster Medical Innovation
Senators Richard Burr (R–NC) and Tom Coburn (R–OK) say that the Obama administration and Congress should cooperate on changes to FDA’s culture so that the United States can continue its leadership role in medical innovation. Writing in an op-ed piece in the Washington Times in February, the senators said that some of FDA’s actions “are eroding America’s leadership in medical innovation. Tragically, reduced innovation not only means a loss of scientific expertise, but results in fewer life-improving drugs and devices for patients.”
They reviewed a study from the Competitive Enterprise Institute showing “how FDA mismanagement can cause ‘deadly delays’ for invisible victims, patients whose names we do not see in the news but whose lives could be improved or extended by a new drug or device.” The study estimated that if FDA’s three-year approval process for a new kidney cancer treatment had been accelerated, as many as 3000 people could have had their lives extended. Burr and Coburn (who is a doctor) also reported that more than two-thirds of doctors say FDA’s approval process is too slow.
“We need an FDA that embraces a culture that places more primacy on the promotion of patient benefit than the status quo,” the senators wrote. “FDA reviewers need to weigh potential dangers from innovative treatments against the potential life-improving benefits to patients. Too many promising treatments are languishing behind mounting rules that cannot be justified by a focus on safety alone.”
They mentioned three areas in which bipartisan congressional cooperation this year would help the agency, as follows:
Members of Congress thoughtfully reauthorize FDA user fees for devices and drugs in a common-sense manner.
Members of Congress overturn the new medical device tax in the Patient Protection and Affordable Care Act that will increase costs and reduce innovation.
Support positive FDA steps in revising the 510(k) medical device pathway.
“If recalibrated,” the senators concluded, “FDA can provide regulatory certainty, free up medical innovation, and strike the right balance between potential risks and patient benefit. This is how we win the future for America’s patients.”
FDA, FTC Duck Jurisdiction over Misleading LASIK Ads
Two years ago, CDRH’s then-director of compliance, Timothy Ulatowski, cautioned the nation’s eye care professionals not to make misleading or unsubstantiated claims in their advertisements about the effectiveness of lasers used in LASIK surgery. Since then, the center has not conducted any follow-up and now has announced it has no jurisdiction over such LASIK ads.
“These lasers,” wrote Ulatowski back then, “are restricted medical devices that have been approved for particular uses and have risks associated with their use. Advertising and promotional materials for FDA-approved lasers used during LASIK procedures must be truthful, properly substantiated and not misleading.”
Fast-forward 20 months to January 28, when CDRH ombudsman David S. Buckles told LASIK victim and activist Paula Cofer: “I’ve investigated the matter you raised regarding the advertising practices cited in your message. Because the advertising practices apparently do not relate to medical products per se, but rather are advertising for the provision of services, we do not have jurisdiction in this matter. My understanding is that these issues are handled as ‘service industry claims’ by the Federal Trade Commission.”
Cofer, an FDA advisory panel patient representative, had sent CDRH compliance director Steven Silverman what she called a “blatantly deceptive” Web site link by Florida-based Brandon Eye Associates. Silverman passed her off to Buckles, who has no enforcement authority.
In her request to Silverman, Cofer wrote that the Web site’s front page banner headline, “Kiss Your Contacts and Glasses Goodbye,” is misleading and deceptive. There is no disclaimer that some patients may need to continue wearing glasses or contact lenses, and there is no mention of risks, side effects, or contraindications.
“This is an example of the typical hype and failure to disclose risks that is prevalent in LASIK advertising on the Internet,” Cofer’s e-mail to Silverman said. “We, the injured LASIK patient community, have complained about this for years, but I have seen no change in the way LASIK is advertised. Former director of compliance, Timothy Ulatowski, sent a letter on 5/22/2009 to eye care professionals in what appeared to be a crackdown on false and misleading LASIK ads. Since that time, it’s only gotten worse.”
Nearly 300 people signed an online forum supporting a petition filed by former CDRH ophthalmic devices director Morris Waxler, seeking an immediate ban on the procedure because of its unacceptable adverse events rate. Another victim and activist, Dean Kantis, says he has sent CDRH “hundreds” of violative LASIK ads, to no effect. Has FDA investigated any of them, and if so, with what result? Buckles says “we are not in a position to disclose the status or even the existence of compliance and enforcement actions that are being contemplated or are in progress.”
Meanwhile, a request by Kantis to FTC that it exercise its jurisdiction brought this diversionary response from deputy compliance director Richard Cleland: “We have regulatory responsibility over many areas and limited resources. In the case of advertising for LASIK surgery, there are other regulators who also have responsibility—local professional practice boards and attorney generals.”
The two agencies’ evasive tactics in this arena are barely background noise to the multi-million-dollar promotional hoopla on the Internet and in local media markets for this procedure.
Finally, research on FDA’s Web site by well-known Washington lawyer and former FDA medical device compliance director Larry R. Pilot has revealed that ambulatory surgical facilities (ASFs) performing LASIK surgery may have a 100% violation rate when it comes to filing mandatory medical device reports (MDRs) with the agency. In a January 28 letter to Buckles, Pilot said he found 38 ASFs that had been inspected by FDA since mid-2009 to the present, 26 of which were sent warning letters.
Pilot told Buckles his “understanding of this summary is that of every LASIK ASF that FDA/CDRH inspected during this two-year period, not one complied with the MDR regulation that has been in effect since prior to the approval of LASIK eye surgery devices.” Pilot said that whether these inspections were taken pursuant to an assignment from CDRH or a specific program issued to the field, “this fact is astounding; namely, that each inspected LASIK ASF was in violation of the MDR regulation since the commencement of their business to provide surgery to their patients.”
Pilot contrasted the latest MDR violation data on ASFs with the situation that existed before public pressure caused FDA to convene a special, two-day meeting of the Ophthalmology Device Advisory Committee on LASIK safety and effectiveness in April 2008. After the meeting, former CDRH director Daniel Schultz “virtually acknowledged that no LASIK ASF had ever been inspected for compliance…yet,” Pilot continued, “in spite of requests to simply visit the nearest LASIK ASF in Rockville [Maryland], it was not until July 2009, more than a year later, that FDA/CDRH visited two nearby LASIK ASFs. Not surprisingly, both of these LASIK ASFs in the same Rockville, MD, location as the FDA commissioner’s office were in continuous violation of law and regulation.”
Pilot’s letter asked Buckles to provide copies of any directive to FDA’s field to inspect ASFs and to say what FDA did with the information from those inspections.
Report on 510(k) Recalls Is ‘Full of Errors’
An Archives of Internal Medicine report by National Research Center for Women & Families director Diane Zuckerman and colleagues on the dominance of 510(k) devices in high-risk device recalls is “full of errors” and should not have passed peer-review, according to Pilot. He said he is planning a rebuttal article.
Pilot, who helped draft the 510(k) law in 1976, said that the following were among the errors he found in the article:
In their first sentence, Zuckerman et al wrote that the 1938 Federal Food, Drug, and Cosmetic Act did not apply to medical devices, when in fact its adulteration and misbranding provisions did apply to medical devices.
The authors wrote that the 1976 Medical Device Amendments established the clinical studies–based premarket approval (PMA) route to market as “similar” to the NDA (new drug application) route for drugs, when in fact PMA was not based on clinical studies and took an “opposite” approach to substantial evidence compared with NDAs.
The authors’ claim that Sec. 510(k) provided an alternative route to market to PMA and that 510(k) “was intended to provide a less-burdensome route to enable newer versions of existing devices to enter the market” is also untrue. The 510(k) was simply a premarket notification of marketing intent, giving FDA 90 days to object and require a PMA submission. Only in the 1990 Safe Medical Devices Act did FDA gain authority over 510(k) device marketing.
Pilot said he found numerous other errors in the article, which he described as an apparent effort to rewrite history, discredit the 510(k) provision, and deflect criticism of FDA’s management of that provision onto the law itself. The Safe Medical Devices Act of 1990, he said, actually converted the 510(k) provision into a “mini-PMA” process that gave FDA the authority to place design, labeling, and marketing requirements on 510(k) devices to justify issuance of a required clearance “order.” The act also gave FDA the power to extend the 90-day automatic marketing deadline for weeks, months, or years until CDRH is satisfied that acceptable substantial equivalence has been established.
Since then, Pilot said, any criticism of the way 510(k) devices perform in the marketplace is a reflection on FDA competence in reviewing and imposing marketing conditions, not on the adequacy of the underlying statutes involved. FDA has all the authority that it needs to better manage 510(k)s—and failures may be due to managerial incompetence, he said. “Didn’t FDA clear [or] approve marketing? What did they miss as part of the PMA or 510(k) review that would have prevented recall?” Pilot asked.
In their report, Zuckerman et al said they analyzed FDA’s list of high-risk device recalls from 2005 to 2009. During this time period, 113 devices were recalled due to their potential to cause serious health problems or death. “Of these, 21 (19%) had been approved through the premarket approval process, 80 (71%) were approved through the 510(k) process, and eight (7%) were exempt from regulation,” the researchers said. “Of the recalled devices cleared for market through the 510(k) process, 12% were marketed for risky or life-sustaining Class III indications, which are required by law to undergo a full premarket approval regulatory review,” they said. The report notes that the most common recalls were cardiovascular devices (31%). Of these, two-thirds (66%) were cleared using the 510(k) process and 12 (34%) were approved through the PMA process.
“FDA’s implementation of the 510(k) process has received considerable criticism from public health advocates and from other federal agencies in reports, medical journal articles, and testimony before Congress,” the authors said. “U.S. courts have also recognized the shortcomings of the expedited process. However, the relatively small division of FDA charged with device approvals does not receive sufficient funding from Congress to conduct premarket approval on every device,” they noted.
The authors say that when devices that were exempt from any kind of review are added to the mix, “they comprise more than three out of four of the high-risk recalls during the last five years.” The report concludes that the standards used to determine whether a medical device is a high-risk or life-sustaining prior to approval are “clearly very different from the standards used to recall a medical device as life-threatening. Our findings reveal critical flaws in the current FDA device review system and its implementation that will require either congressional action or major changes in regulatory policy.”
TMJ Implant Makers Ordered to Conduct Studies
To better understand why and when temporomandibular joint (TMJ) implants are removed or replaced, FDA has ordered three TMJ implant makers to conduct postmarket surveillance studies. The studies will examine the length of time before the implants are removed or replaced due to pain or other reasons. The agency says that TMJ Solutions, TMJ Medical, and Biomet Microfixation make all of the currently approved TMJ devices marketed in the United States, and they have 30 days to submit a study plan for approval.
The agency says it recently reviewed TMJ implant–related adverse-event reports submitted between 2004 and 2010 and found “a substantial number of patients who had implants replaced within three years or less after implantation because of extreme pain. This is considerably shorter than the expected minimum five-year life span of the device, based on premarket mechanical testing.”
FDA says it currently is not recommending any changes on TMJ implant use, but it may “revise its recommendations or issue other recommendations” after reviewing new clinical data from the postmarket studies. As part of its review, the agency says it will “consider whether labeling changes, additional preclinical and clinical testing requirements, or other regulatory actions are necessary for these devices.”
About the Author(s)
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

