The Medtech Marketplace in 2007 13047
Technological innovation and corporate consolidation are altering the competitive landscapes of medtech’s hottest sectors.
January 1, 2007
BUSINESS PLANNING & TECHNOLOGY DEVELOPMENT
|
In 2007, market growth and technological advances in the medical device arena show no signs of slowing. Established sectors such as cardiovascular and orthopedics continue to see new growth engines on their horizons. Meanwhile, sectors such as neurotechnology are only beginning to realize the potential of emerging technologies.
This article takes a look at what's on the horizon for some of medtech's key sectors this year and beyond.
Radical Change in IVDs
The global market for in vitro diagnostics (IVDs) is estimated to have exceeded $30 billion in 2005 and was on course to reach $32 billion in 2006. Although overall growth remains in single digits at around 7%, the IVD market is entering a new era in which new technologies and new players will radically change the industry landscape and drive sales to more than $40 billion by 2010.
In 2006, the IVD market saw the entry of a new major player with the acquisition of Diagnostic Products Corp. (DPC; Los Angeles) and the diagnostics division of Bayer HealthCare (Leverkusen, Germany) by Siemens AG (Erlangen, Germany). The medical imaging giant paid ?4.2 billion (US$5.3 billion) for the Bayer business and $1.86 billion for DPC. The newly created Siemens Medical Solutions Diagnostics will be number two in the worldwide immunodiagnostics market.
In 2005, Roche Diagnostics (Indianapolis) had an estimated 20% market share, followed by Abbott (Abbott Park, IL) with about 12%.
Although Siemens dominated the mergers and acquisitions news in the industry in 2006, another deal completed during the year saw Becton Dickinson (BD; Franklin Lakes, NJ) expand its presence in the cancer diagnostics market through its $350 million acquisition of TriPath Imaging (Burlington, NC). The deal followed the completion of BD's acquisition of GeneOhm Sciences (San Diego) in early 2006, which gave BD a foothold in the emerging field of healthcare-associated infections. BD also decided to quit the increasingly competitive blood glucose– monitoring market. Its monitors and test strips generated revenues of about $105 million in fiscal 2006.
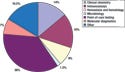
Accounting for an estimated 7% of the global IVD market in 2005, molecular diagnostics is the fastest-growing segment of the industry (see Figure 1). From a base of $2.6 billion in 2005, it is projected to grow at a compound annual growth rate (CAGR) of 14% over the next five years.
Point-of-care tests are also driving market growth. Accounting for a third of the global IVD market at almost $12 billion in 2005, tests used at the patient's bedside, in doctors' offices, and by the patient at home are forecast to grow at a CAGR of 7.8% over the next five years.
Technology innovations are increasing the pace of change, producing tools that enable earlier and more-accurate diagnosis of disease, improved clinical decisions, and better monitoring of treatment. Adoption of innovative technologies in the marketplace, however, will need to overcome hurdles in reimbursement and regulatory policies, which are not keeping pace with the development of innovation.—Jeanette Marchant, principal, JM Communications
Orthopedic Opportunities Still Robust
While annual growth rates for the orthopedics sector have decelerated somewhat from the near-20% levels of a few years ago, the sector is still setting a robust pace. Worldwide sales of orthopedic products in 2005 generated revenues of $25.9 billion, with every major segment reporting double-digit growth. Such growth was led by the spine segment with an 18.9% increase over 2004. Overall, 2005 sector revenues topped year-earlier performance by 12.7%.1 Market estimates placed 2006 global revenues for the sector around $28.2 billion.
Merger and acquisition activity in the orthopedics sector was down in 2006 compared with the previous year. Nevertheless, the sector's major players produced a big surprise at year's end. Shortly after Smith & Nephew acknowledged that the company had made a bid for Biomet in early November, offers from other suitors apparently upped the ante, causing Smith & Nephew to exit negotiations. A group of private equity investors—including Biomet founder and former CEO Dane Miller, who was forced out of the company in March 2005—emerged as the new buyers.
Although the reconstructive-device and joint-replacement implant segment has experienced slowing growth over the past two years, it continues to lead the orthopedics sector with 2005 sales of $9.6 billion and a 37.1% share of overall revenues.
Spinal implants and instrumentation accounted for 16.7% of the global market, while fracture repair took 11.6%, orthobiologics took 9.3%, arthroscopy and soft-tissue repair took 8.5%, and other orthopedic products took 16.7% (see Table I).
Orthopedic Product Segment |
Reconstructive devices and joint replacements |
Spinal implants and instrumentation |
Fracture repair |
Orthobiologics |
Arthroscopy and soft tissue repair |
Other orthopedic products |
Total |
Table I. Global orthopedic revenues for 2005, by product segment. Figures may not add up due to rounding. Source: The Worldwide Orthopedic Market: 2005-2006, Knowledge Enterprises Inc. |
The spine sector continues to be orthopedics' hottest segment. While it too has slowed from the torrid pace of a few years ago, when year-over-year growth was pushing 30%, “It's still the place to be,” says John McCormick, medtech analyst and a managing director at Healthpoint Capital LLC (New York City), an investment banking firm specializing in orthopedics.
With knee and hip replacements no longer commanding their premium prices, the small-bone segment is showing increasing promise as a new growth area for joint-replacement technologies. “Since we launched Small Bone Innovations in early 2005, about 25 to 30 new companies have been formed that focus on this area,” says John Viscogliosi of Viscogliosi Brothers LLC (New York City), an investment banking firm that is focused on specialty orthopedics.
By 2010, the orthopedics sector is expected to produce $44.7 billion in global revenues, an increase of more than 166% over the seven-year period beginning in 2003. Growth in 2006 was estimated to be about 12%. Going forward, an uptick to a 15% annual growth rate is forecast through 2010.2—Art Kerley, president, Fairfield Factor Inc.
Cardiovascular Opportunities and Obstacles
With a global valuation of about $27 billion, the cardiovascular market is currently growing at an annual rate of 16%.3 In the North American market, cardiovascular devices generated revenues of $17.88 billion in 2005. With an aging population that has a greater incidence of cardiovascular disease as a key driver, the North American cardiovascular market is expected to reach $40.46 billion by 2011.4
Stent Evolution. The worldwide drug-eluting stent market is currently valued at about $6 billion.3 However, the good times for drug-eluting coronary stents were seriously challenged last year following clinical presentations at the 2006 World Congress of Cardiology, in Barcelona, Spain. The findings, which suggested a significant risk of late-term thrombosis associated with the devices, were widely and prominently reported in industry, business, and popular media. However, clinical studies reported at the Transcatheter Cardiovascular Therapeutics (TCT) conference in October and a meeting of FDA's circulatory devices panel in December gave drug-eluting stents a new lease on life. The panel members expressed concern about reported extensive off-label use of the devices. They also called for increased postmarket studies and surveillance. But they did not recommend any significant changes in clinical trials or the premarket approval process for new drug-eluting coronary stents seeking entry to the U.S. market.
Drug-eluting stents poised for FDA approval later this year or in early 2008 include Endeavor from Medtronic Inc. (Minneapolis); Xience, manufactured by Abbott (Abbott Park, IL); and CoStar from Conor MedSystems Inc. (Menlo Park, CA). Conor has recently agreed to be acquired by Cordis Corp. for $1.4 billion.
CRM Potential. The U.S. cardiac rhythm management (CRM) market produced revenues of $5.99 billion in 2005. The U.S. CRM market is forecast to reach $16.79 billion by 2012.5
CRM devices are thought to have significant potential for market growth outside the United States. In Europe, where adoption rates have lagged, markets for the devices are now poised for significant growth. Asian markets—China, in particular—are expected to see gains as well.
Valve Stability. In the heart valve segment, biological technologies continue to make inroads in the $1 billion market.6 Yet mechanical prosthetic devices still dominate the market with a 60% share.
Percutaneous heart valve technology, which enables the interventional implantation of devices without the need for major surgery, is currently one of the most intensely researched product development areas in the cardiovascular sector. By opening the replacement valve market to patients deemed unsuitable for open-heart surgery, percutaneous and other novel valve technologies are expected to provide significant growth opportunities going forward.7
Continued Assistance. Heart-assist devices represent one of the smallest segments of the cardiovascular sector. While a great deal of the activity in the segment is experimental, there are a number of commercially available ventricular-assist devices on the market, which is expected to reach $350 million in the United States by 2009.8
With cardiovascular disease accounting for the greatest outlay of healthcare expenditures, the sector presents a dynamic opportunity for medtech manufacturers to develop diagnostic and therapeutic devices that are less invasive, enable shorter hospital stays, and speed patient recovery—all while reducing costs for patients, payers, and providers.—Art Kerley, president, Fairfield Factor Inc.
Significant Potential in Neurodevices
The neurodevice market is one of the fastest-growing sectors of the overall medical device industry. Global neurodevice sales in 2005 were approximately $3.4 billion, representing growth of about 21% over the prior year.9 The neurodevice sector consists of four segments: neuroprosthetics, neurostimulation, neurosurgery, and neurofeedback (see Table II).
Segment |
Neuroprosthetic |
Neurostimulation |
Neurosurgical |
Neurofeedback |
Table II. Descriptions and global revenue estimates of neurodevice segments. Source: NeuroInsights. |
Neuroprosthetics are electromechanical devices that interface with the nervous system to compensate for a sensory deficiency or motor deficiency. The neuroprosthetics market is currently dominated by cochlear implants, which had estimated worldwide sales of $450 million in 2005, representing 21% growth over 2004.9
Neurostimulation devices generated sales of $1.2 billion in 2005 with 16% growth.10 Currently, most revenue in this sector comes from patients with chronic pain, movement disorders, and epilepsy. In the next three years, patients with severe depression, migraine headaches, and obsessive-compulsive disorders can expect neurostimulation devices to be approved by FDA. New neurostimulation treatments for patients with chronic anxiety, obesity, bulimia, and Alzheimer's disease are a bit further out on the horizon.
The market for neurosurgical medical devices totaled roughly $1.75 billion in 2005, and is projected to grow at a rate of 20% annually.9 This category includes minimally invasive surgical tools, surgical navigation devices, neurovascular interventions, and other medical supplies for neurological diseases—such as shunts for hydrocephalus and peripheral-nerve repair guides.
The convergence of software, imaging, and brain research is leading to new neurofeedback and software-based solutions for a wide range of cognitive, emotional, and sensory disorders. If companies in this space can demonstrate efficacy and connect with customer concerns about side effects from neuropharmaceuticals, they have the potential to become first-line treatments for disorders like attention deficit hyperactivity disorder (ADHD) and mild cognitive impairment.
The number of neurodevices registered with FDA across all segments rose more than 400% from 1998 to 2005, compared with only a 16% increase for overall medical device registrations.9 Advances in technology paired with increased market uptake are driving sustained market growth of neurodevices and penetration into new disease indications, including stroke, obesity, and depression. Critical industry challenges facing neurodevice companies include patient bias against implantation, reimbursement, and the need to educate clinicians. —Zack Lynch, executive director, Neurotechnology Industry Organization (San Francisco).
Imaging Opportunities, Big and Small
In the United States, market demand for medical imaging equipment is expected to reach more than $16 billion by 2010, with a forecast CAGR of 6.8% between 2005 and 2010. By 2015, U.S. market demand for imaging equipment is expected to top $21 billion.
Computed tomography (CT) is expected to lead the sector's growth, with a CAGR of 10.3% between 2005 and 2010. By 2010, U.S. market demand in the CT segment will reach $3.3 billion (see Table III).11
Medical Imaging Segment |
Computed tomography |
Magnetic resonance imaging |
Medical x-ray |
Nuclear medicine |
Ultrasound |
Fluoroscopy |
Positron emission tomography |
Other equipment |
All equipment |
Table III. Actual and forecast U.S. market demand and compound annual growth rates for medical imaging equipment, 2000–2015, by segment. Source: The Freedonia Group Inc. |
The annual meeting of the Radiological Society of North America (RSNA) in late November is the traditional launchpad for products in every imaging technology. Devices, applications, and news emerging from the event provide a solid gauge of the current state of the imaging sector, as well as its future directions. At the 2006 RSNA conference, for the second year running, molecular imaging was a major undercurrent at the show. Capable of providing images at both the cellular and molecular levels, molecular imaging technologies provide a functional view of the body compared with traditional modalities that reveal internal structures.
Developments in image management and information technology were also front and center at RSNA 2006. As the picture archiving and communications systems (PACS) market matures, vendors are moving to consolidate radiology information systems (RIS) and PACS into integrated systems.
Another underlying theme at RSNA was the strategic targeting of unmet or poorly met niche markets by start-up companies. Hand-carried ultrasound vendor SonoSite (Bothell, WA) is an example of an imaging company that has found its niche.
The company targets nonspecialist clinicians using ultrasound at the point of care in emergency departments, intensive care units (ICUs), and operating rooms. SonoSite has shipped 3000 MicroMaxx ultrasound systems since June 2005, when it released its third-generation mobile system.
Another new-modality product on display at RSNA was the CereTom, an air-cooled, battery-powered (and wireless) portable CT system. Manufactured by NeuroLogica (Danvers, MA), the CereTom targets head and neck imaging. Identifying neuro-imaging as an underserved market segment, NeuroLogica CEO Eric Bailey says, “Only 15% of CT exams are of the head, and the big modality vendors never call on neurologists or neurosurgeons.”
Overall, imaging technology manufacturers at RSNA 2006 demonstrated solid progress, with top vendors largely focused on advancing their existing products.
At RSNA, Siemens took computed tomography to the next level with its 128-slice Somatom Definition. The system uses two 83-millisecond x-ray tubes, enabling the system to complete a 128-slice study in 164 milliseconds—or a 64-slice study in roughly half the time (83 milliseconds). In cardiology studies, a shorter data acquisition time makes it possible to generate good images at a higher heart rate, a capability that can mean less medication required for patients undergoing cardiac CT exams.
Toshiba America Medical Systems (Tustin, CA) used the 2006 RSNA meeting to show off its 256-slice CT scanner as a works-in-progress technology. Toshiba estimates that a 256-slice scanner is two years from market.
Philips Medical Systems (Bothell, WA) introduced the iU22 high-end ultrasound system, which uses iSlice, the company's proprietary volumetric data scheme. The iSlice system includes a new 4-D cardiac scan head, the X7-2 x-matrix array, and QLAB workstation.
At RSNA 2006, GE Healthcare launched the LightSpeed VCT XT volume CT scanner, which targets cardiology imaging, reduces a patient's radiation exposure by up to 70% for diagnostic cardiac scans and is capable of capturing images of the heart and coronary arteries in as few as five heartbeats.
Overall, RSNA 2006 saw solid progress in the field of imaging—much of it proprietary in nature. The largest vendors leveraged their size and access to capital by advancing technology on big-ticket items. Many of these new offerings compete as much based on their proprietary technology as they do by offering truly superior solutions. Meanwhile, smaller vendors demonstrated their ability to find and quickly capitalize on market opportunities overlooked by larger vendors.—Tim Gee, principal, Medical Connectivity Consulting
References
1. The Worldwide Orthopedic Market: 2005– 2006 (Chagrin Falls, OH: Knowledge Enterprises, 2006).
2. 2006 Orthopedic Industry Forecast (New York: Healthpoint Capital, 2006).
3. “Boston Scientific Corporation Analyst Meeting,” Boston Scientific Web site, Investor Relations, Webcasts & Presentations [online] (Natick, MA: Boston Scientific, 6 November 2006 [cited 5 January 2006]); available from Internet: http://phx.corporate-ir.net/ phoenix.zhtml?c=62272&p=irol-presentations.
4. North American Cardiovascular Devices Market—Investment Analysis and Growth Opportunities (San Antonio, TX: Frost & Sullivan, 2006).
5. U.S. Cardiac Rhythm Management Market (San Antonio, TX: Frost & Sullivan, 2006).
6. “Sealants, Glues, Adhesion Prevention Expecting Double-Digit Growth Innovations in the $1+ Billion Heart Valve Market,” MedMarkets (Foothill Ranch, CA: MedMarket Diligence, 2006).
7. U.S. Markets for Heart Valve Devices 2006 (Toronto: Millennium Research Group, 2006).
8. U.S. Markets for Cardiac Assist Devices (Toronto: Millennium Research Group, 2005).
9. The Neurotechnology Industry 2006 Report: Market Analysis and Strategic Investment Guide of the Global Neurological Disease and Psychiatric Illness Markets (San Francisco: NeuroInsights, 2006).
10. “Emerging Treatments for Epilepsy and Seizure Disorders,” Neurotech Insights: The Neurotechnology Industry Newsletter 2, no. 6 (July 2006): 1, 9–11.
11. Medical Imaging (Equipment, Agents & Consumables) (Cleveland: Freedonia Group, 2007).
Copyright ©2007 MX
About the Author(s)
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

