A Better See-and-Do Technology for Bland Embolization Procedures
Recently FDA-cleared Easi-Vue embolic microspheres are nondeformable and radiopaque and feature a proprietary delivery system that enables controlled metering of the microspheres into a patient.
September 30, 2022
In bland embolization procedures, a variety of embolizing agents can be delivered through the tumor’s feeding artery to occlude its blood supply. However, the current offering of common embolic microspheres that are available to an interventional radiologist cannot be seen under x-ray, said Gary Donofrio, chief business officer at ABK Biomedical, in an interview with Design News, MD+DI's sister publication.
This is a problem, he said, because, “the interventional radiologist forms the sort of practice around being able to see and do using x-ray-based technologies, but this is one form of therapy where they don’t have direct visualization.”
Donofrio went on to say this is what sparked one of the founders of ABK Biomedical to create a radiopaque embolic microsphere. “Physicians had an indirect visual signal using contrast medium during injection,” he said. “What we’re doing is enabling them to have a better direct see-and-do technology.”
ABK Biomedical’s Easi-Vue embolic microspheres are made of glass that contains radiopaque elements that company PhD scientists developed through experimentation. Reagents are blended and then melted in a high-temperature furnace. After the glass is quenched, it is ground into small glass particles and then spheroidized into microspheres, explained Anthony Headley, ABK Biomedical’s VP of manufacturing, in the same interview.
Because they are made of glass, Easi-Vue microspheres cannot compress. “They are like tiny marbles, versus other [microspheres] being like tiny little gummy balls that squeeze down,” Donofrio said. “Easi-Vue will stay true to size,” he explained. “We're striving to give physicians more control by allowing them to choose a calibrated sized microsphere that won’t change shape or size inside the patient.”
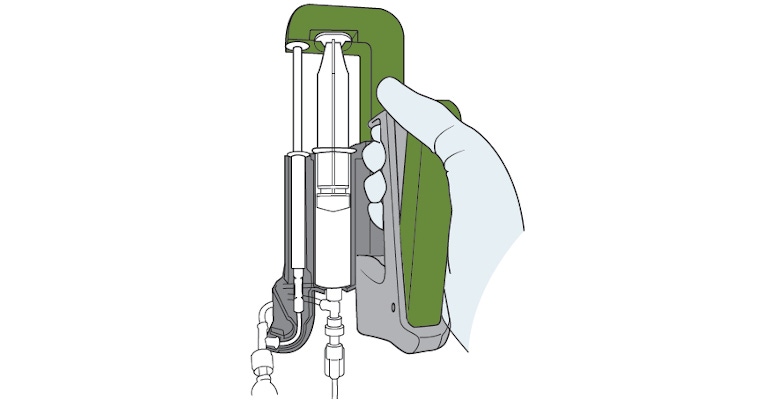
Easi-Vue embolic microspheres also feature a proprietary delivery system. Introducing other embolic microspheres requires the doctor to oscillate between a syringe filled with saline and one filled with the microspheres to suspend the microspheres in the saline. “And once [the physician] feels like they’re adequately mixed, they’ll connect it to a microcatheter,” Donofrio said, noting that there is no real accuracy as far as the concentration of microspheres in any aliquot of fluid. “As they inject them, part of the injection fluid could be concentrated with a lot of microspheres, and part of it may be less so as they start to settle out and become more fluid-based.”
Easi-Vue’s delivery system works by using two syringes, one filled with saline and the other with the microspheres. “We have a control handle that depresses both syringes at the same time and the saline-filled syringe starts the fluid path into the patient through the microcatheter, and the other syringe then begins to drop the microspheres into the fluid path into the patient,” Donofrio said. “It's a controlled metering of microspheres into the delivery fluid into the patient.”
ABK Biomedical has recently received FDA clearance for the use of Easi-Vue embolic microspheres in treating hypervascular tumors and arteriovenous malformations. Donofrio said he expects the microspheres to be put into clinical practice by the end of October, in a limited release fashion.
About the Author(s)
You May Also Like




