The Top 10 510(k) Trends Since 2000
March 14, 2016
Although the average time for FDA to clear a 510(k) application has been trending down in recent years, it had increased by 60% from 2000 to 2010, increasing from an average of 96 days in 2000 to 154 in 2010. Interestingly, the FDA's data indicates that the delay was largely a result delays on the part of the submitter.
Brian Buntz
The clearance process, established under the Medical Device Amendments of 1976 and reformed in the 1990s, provides a way for medical device manufacturers to get to market than the more-stringent PMA requirements by claiming similarity to a predicate medical device. Medical device safety activists claim that it too often allows unsafe devices to make it to market and, in 2011, the Institute of Medicine reached a similar conclusion after FDA commissioned them to study potential reforms.
It is worth noting that FDA this year has issued orders to require PMAs for two types of initially 510(k)-cleared devices that have proved especially problematic: metal-on-metal hip implants and vaginal mesh. A recent U.S. Senate report also faulted the 510(k) process for failing to catch reprocessing problems with a type of endoscope, called a duodenoscope, linked to deadly superbug outbreaks.
For now, the overall trend since 2000 has been for FDA to spend more time scrutinizing applications, according to independent analyst Steven Gill, whose research on 510(k) trends was used extensively in this feature--along with information from FDA itself and from Emergo. Let us know if you would like to see additional research relate to the 510(k) process.
Here are 10 510(k) trends especially worth noting:
1. The Time Taken for FDA to Clear a 510(k) Application Increased 60% in a Decade
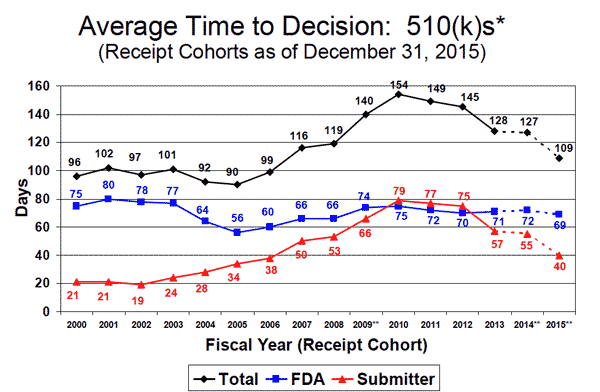
Although the average time for FDA to clear a 510(k) application has been trending down in recent years, it had increased by 60% from 2000 to 2010, growing from an average of 96 days in 2000 to 154 in 2010. Interestingly, the FDA's data indicates that the delay was largely a result delays on the part of the submitter. In roughly that same time period, however, there was also an increase in requests from FDA for more information during the 510(k) review process.
According to FDA's most recent numbers, the average time to clear a 510(k) submission was 109 days in 2015.
According to Emergo, the average time for FDA to process a 510(k) application was just 39 days from 1976 to 1980, 61 days from 1981 to 1985, and 83 days from 1986 to 1990. It reached an unprecedented peak--an average of 173 days--from 1991 to 1995.
2. Radiological Instruments Took FDA the Longest to Process
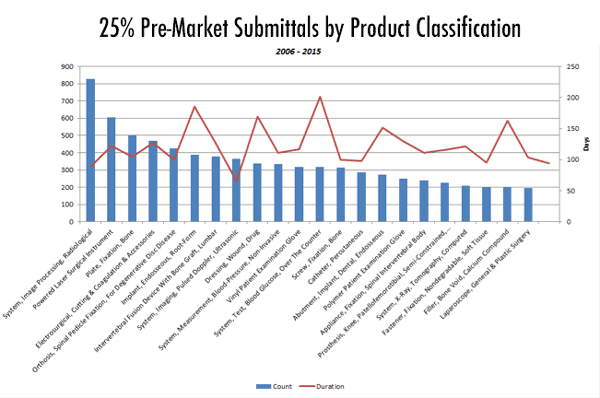
The product category of radiological image processing systems took FDA considerably more time to process than other categories -- an average of more than two years (more than 800 days). By contrast, FDA processes products such as laparoscopes and bone fillers in about 50 days.
3. FDA Is Deeming More 510(k) Applications Substantially Equivalent
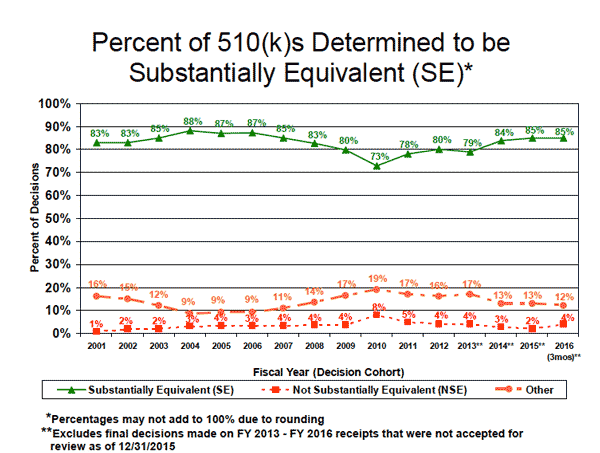
Since 2010, FDA has been deeming more 510(k) applications to be substantially equivalent--roughly 85% in 2015 versus 73% in 2010. In the early 2000s, however, the agency had been finding more devices to be substantially equivalent than it does today. In 2004, for example, it ruled that 88% of 510(k) submissions were substantially equivalent.
4. The Volume of 510(k) Submissions Appears to Be Holding Steady
The number of annual submittals and 510(k) clearances has been mostly constant over the past decade although the volume of submittals ticked upwards significantly in 2006.
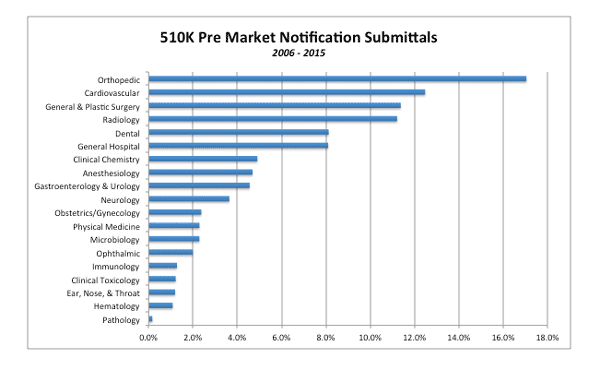
5. The 510(k) Process Is Used More for Orthopedics Than Any Other Product Type
6. Fewer U.S. Companies Filed 510(k)s in 2015
Last year, fewer domestic medical device companies made 510(k) submissions than any year since 2011. As reported last month by Emergo, less than two-thirds of 510(k) applications that were cleared by the FDA whereas 78% of 510(k) applications in the prior year were. Less than 65% of 510(k) applications cleared by the agency in 2015 were filed by US companies, down significantly from 78% of applications in 2014.
7. FDA Is Requesting More Supplementary Information
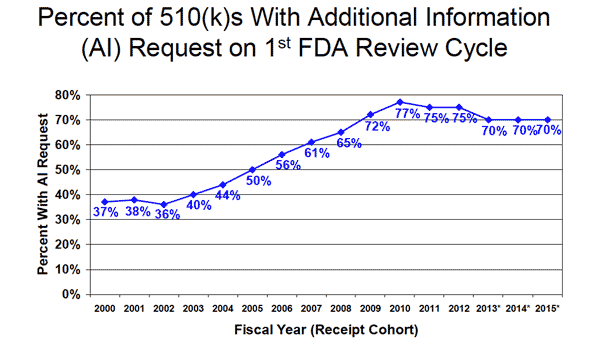
FDA is now making roughly twice as many requests for additional information on the first FDA review cycle than it did in the year 2000, although requests for additional information during the second FDA review cycle have fallen significantly from 35% in 2010 to 6% last year.
Information above pulled from FDA report titled "1st Quarter FY 2016 Package (in lieu of meeting) MDUFA III (FY 2013-2017) Performance."
8. The Volume of 510(k) Submissions Growing Faster than 510(k) Clearances

While the volume of 510(k) submissions has increased considerably in the past decade, the level of 510(k) clearances granted is mostly flat.
9. The Vast Majority of 510(k)s Are Cleared within 300 Days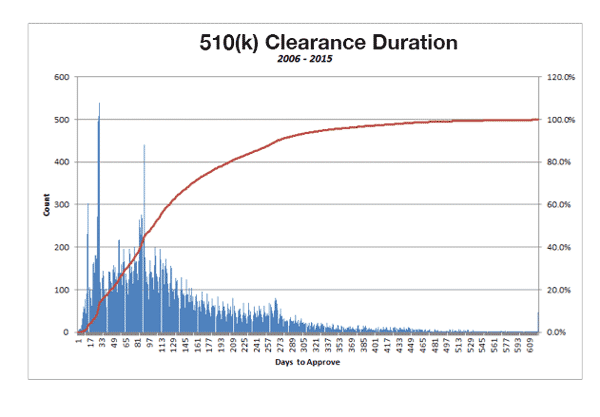

While a considerable number of 510(k) submissions are cleared in less than a month, the majority are cleared in under 300 days. Although some devices take more than 600 days to win clearance, these are a special case.
10. Third-Party Review Speeds Things Up Considerably
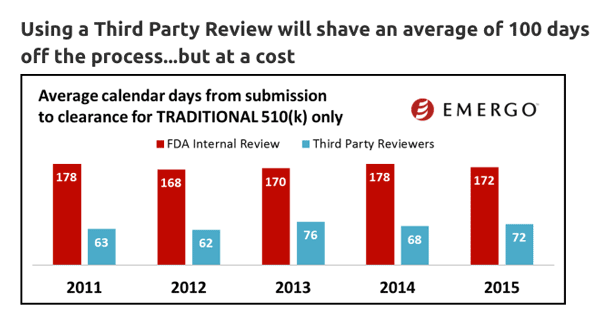
According to research from Emergo, using a third-party review can shave an average of 100 days off the 510(k) process. While third-party reviews are not subject to the FDA user fees, it is generally substantially more expensive to use a third-party for 510(k) submissions than to file directly with the FDA.
Learn more about cutting-edge medical devices at BIOMEDevice Boston, April 13-14, 2016. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
About the Author(s)
You May Also Like


