PMA, 510(k) User Fees Increase 8.5%
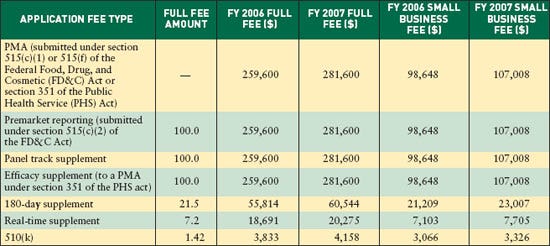
NEWS TRENDS As required by the Medical Device User Fee Stabilization Act of 2005 (MDUFSA), premarket approval (PMA) applications and 510(k) user-fee rates for fiscal year 2007 increased less than 10% over FY 2006.The PMA fee jumps from $259,600 to $281,600, an increase of almost 8.5%, the maximum allowed under MDUFSA (see Table I). For businesses with less than $100 million in annual revenues, the fee jumps from $98,648 to $107,008.
October 1, 2006
The 510(k) fee increases from $3833 to $4158, also a near 8.5% increase. The jump for small businesses is from $3066 to $3326.
Fees for premarket reports, panel-track supplements, and efficacy supplements remain the same as for regular PMAs. Fees for 180-day supplements remain at 21.5% of the PMA fee, or $60,544. Fees for real-time supplements remain at 7.2% of the PMA fee, or $20,276.
This is the final year of the 8.5% maximum increase. The FY 2008 fees will be determined by whatever user-fee scheme is agreed upon in the reauthorization of the Medical Device User Fee and Modernization Act of 2002 (MDUFMA), which must be in place by October 1, 2007.
Work has begun on drafting new legislation, but what kind of progress has been made so far is unknown. According to one party involved in the negotiations, the stakeholders involved have agreed not to make public comments about the process.
MDUFSA was enacted after user-fee increases for the first few years under MDUFMA were much steeper than expected. A major problem was Congress failing to appropriate all of the increase it promised for CDRH's budget.
|
Table I. Fee types, percent of PMA fee, and fee rates for MDUFSA, FY 2006 and FY 2007. |
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like