Medtronic Hit With Class I Recall Involving Steerable Sheath
March 18, 2015
In four cases, clinicians observed debris that appeared to come from the device, according to FDA.
Chris Newmarker
|
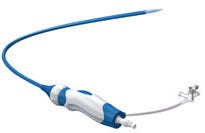
Medtronic's FlexCath Select Steerable Sheath, as shown on the company's website |
FDA has designated as Class I a Medtronic recall involving the company's FlexCath Select Steerable Sheath (10 Fr Select).
The single-use device is used for percutaneous catheter introduction into the vasculature and chambers of the heart.
Medtronic initiated the recall last month after four reports of clinicians observing debris appearing to originate from the hemostasis valve on the proximal end of Model 990065 of the device, according to FDA. There have been no reports of patient injury or death, nor are any predicted, but potential debris in the heart nevertheless poses a risk of serious injury or death.
In a hand-delivered letter to U.S. customers, Medtronic has requested that they return the affected product.
More information about the recall is available on FDA's website.
Material separation appears to be the most frequent problem involving catheter introducers, according to FDA databases.
When Medtronic announced FDA clearance of the FlexCath Advance Steerable Sheath in early 2013, it touted it as a second-generation sheath with an increased degree of deflection and response. The FlexCath, according to Medtronic, provides greater ease compared to the previous generation in reaching the inferior veins of the heart.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like