How a Tampered MRI Sparked a Huge GE Recall
February 27, 2015
The recall involves every MRI system with superconducting magnets that GE Healthcare has ever made--nearly 13,000 of them going back to 1985.
Chris Newmarker
|
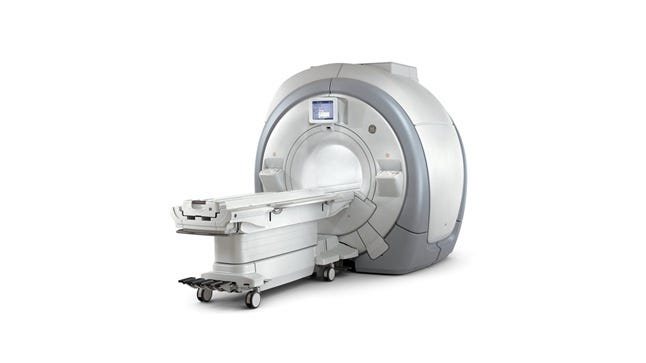
GE's Optima MR450w was one of the MRI systems implicated in the recall. |
FDA recently designated as Class I a massive GE Healthcare recall related to the erroneous disabling of an MRI system's magnet rundown unit, eliminating a method to quickly shut the system down in an emergency situation.
Incidents involved some MRI systems in India, and involved either service personnel or equipment users disabling the magnet rundown unit, according to a GE Healthcare news release.
The Mumbai Mirror had a different take on the situation back in November, reporting that GE engineers told an inquiry committee set up by Tata Memorial Hospital that this switch was removed from all the company's MRI machines in India. The switch was removed after a helium gas release at one of the centers caused by a patient fiddling with the switch.
A delay in shut off, however, can potentially result in life-threatening injuries when metal objects are brought into a magnetic field around an MRI. The FDA says there were two reported injuries when hospital employees entered an MRI room carrying a metal container. The Mirror reports employees at Tata Memorial Hospital remained stuck to an MRI machine for four hours.
The recall involves all GE Healthcare MRI systems with superconducting magnets. In all, more than 30 MRI models were recalled.
GE Healthcare has sent an Urgent Medical Device Correction letter to all its customers informing them of the danger, and instructing them to perform a short test that confirms if the magnet rundown unit is functioning properly and has not been disabled.
The letter also says a GE Healthcare service representative will visit to inspect the Magnet Rundown Unit.
"If the MRU test does not perform as described in each of the 4 steps above, with the specified LED lighting in each step, GE Healthcare strongly recommends that you stop using the system, and immediately call your GE Healthcare representative," - See more at: http://www.diagnosticimaging.com/mri/everything-you-need-know-about-ge-mri-recall#sthash.nI5pmLI7.dpuf
"If the MRU test does not perform as described in each of the 4 steps above, with the specified LED lighting in each step, GE Healthcare strongly recommends that you stop using the system, and immediately call your GE Healthcare representative," the letter states. - See more at: http://www.diagnosticimaging.com/mri/everything-you-need-know-about-ge-mri-recall#sthash.nI5pmLI7.dpuf
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like