Cook Medical among Companies with Serious Recalls
September 5, 2014
A Cook Medical vascular retrieval snare with snare loop detachment issues and faulty packaging on Customed surgical packs are among the recalled medical device products recently receiving a Class I designation from the FDA.
|
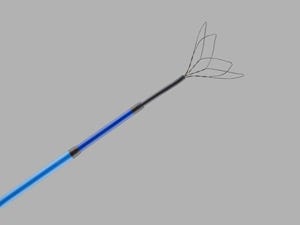
CloverSnare 4-Loop Vascular Retrieval Snare, as shown on Cook Medical's website. |
In the case of the Cook retrieval snare, FDA officials think nearly 700 of the CloverSnare 4-Loop Vascular Retrieval Snares distributed nationwide could cause serious injury or death because a loop separating from a shaft could block blood vessels or even become stuck in the heart or lungs.Four injuries have been reported.The snare is used to manipulate and retrieve items in the cardiovascular system, such as temporary implanted devices and even broken guidewires, coils, balloons, and catheters. The Cook Medical recall affects products manufactured between August 2012 and August 2013 and distributed between March 8, 2013 and July 1, 2014. More information, included affected lot numbers, is available in Cook's recall announcement, and on the FDA's website.The recall of Customed's sterile convenience surgical packs is due to issues around the packs adhering to one another in shipping cases. Separating the bags can cause the plastic film to tear, compromising the sterility of the contents of the surgical instruments inside, according to an FDA announcement.The Customed recall involves all lots manufactured between January 9, 2009 and May 20, 2014. The FDA Class I designation announcement lists a Fajardo, Puerto Rico address for Customed.
Refresh your medical device industry knowledge at MEDevice San Diego, September 10-11, 2014. |
Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like