Schultz Makes Postmarket Issues a Priority for CDRH
Originally Published MDDI May 2006 News Trends
May 1, 2006
News Trends
|
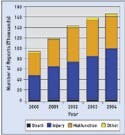
Figure 1. Medical device reports are increasing. CDRH receives about 180,000 reports annually. |
Attendees at AdvaMed's annual meeting in March expected CDRH Director Daniel Schultz to emphasize postmarket issues in his presentation. He did not disappoint.
Two of the major priorities Schultz identified for CDRH in fiscal year 2006 are to “increase our ability to identify, analyze, and act on postmarket information” and to “increase communication of risk-benefit issues to all of our stakeholders.”
Medical device reports have grown from fewer than 100,000 in FY 2000 to a projection of almost 180,000 in FY 2005 (see Figure 1). While an increase in reports doesn't necessarily mean an increase in the actual number of adverse events, the numbers underscore the importance of postmarket surveillance, Schultz said.
However, recalls, a solid indicator of serious problems, are up too, he said. In FY 1998, there were 336 recalls, with five being Class I recalls, which pose a significant public health issue. In FY 2004, there were 638 recalls, 23 of which were Class I.
CDRH faces challenges in dealing with these issues, he said. Device technology is getting more complex, yet the center has limited field resources. Implanted devices, which pose the highest risk, are being used for longer periods and in younger patients. And it is difficult to communicate risk-benefit information in a way that is both timely and balanced.
Hence, CDRH wants to strengthen condition-of-approval studies, improve the MedSun targeted surveillance program, and improve automated information systems, Schultz said.
“The degree of fragmentation that we encounter with postmarket data needs to be corrected,” he said. “We want to develop a culture of collaboration within the center and outside. We need better data sources. A lot of what we get is paper based.”
These will be some of the missions of CDRH's Postmarket Transformation Initiative, announced at the beginning of 2006. Schultz named three people to head the initiative. They are Steve Niedelman of the Office of Regulatory Affairs; Elizabeth Jacobson, a former CDRH and AdvaMed official; and Jeff Brinker, a cardiologist at The Johns Hopkins University.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)
.gif?width=300&auto=webp&quality=80&disable=upscale)