Keys to Market Success Evolving, Panelists Say
Originally Published MDDI July 2006NEWS TRENDS Erik Swain
July 1, 2006
NEWS TRENDS
|
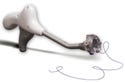
The SuturTek 360° Fascia Closure Device is a replacement for hand suturing. The device represents a major advance in the standard of care. |
What does it take for a small company to succeed in the vast, uncertain medical device market? It's no longer as simple as having a new technology in a fast-growing market, says a panel of experts. Everything from clinical trials to manufacturing must be conceived of differently.
“Whatever data are required by FDA for approval are usually less than the data you have to have when you try to sell the product,” says Mark Speers, managing director of Health Advances (Weston, MA). “The mentality is that success equals [FDA] approval, and you spend money on what it takes to get approval. But that just means you've met the clinical endpoints FDA wants to see. But physicians and payers want to see more data, or different data from what FDA requires. The device industry has traditionally seen this kind of trial as exorbitant. But the good news is that because companies are already doing some trials for FDA, a lot of the time they can add clinical endpoints to the protocol or get the patients to agree to be followed for longer to strengthen longevity data.”
Speers and the other panelists spoke at the Massachusetts Institute of Technology (MIT) Forum of Cambridge, MA, in May, and to MD&DI before and after the event. They emphasized the importance of securing the right people and partnerships early on.
“Regulatory and clinical expertise is essential to making a company successful,” says Sam Navarro, managing director of healthcare investment banking at Cowen and Company, LLC (New York City). “Many companies fail because they don't get powerful and demonstrative clinical data, or they don't execute their clinical strategy very well.”
The right partnerships, from distributors to contract manufacturers, are essential to success, says John Lamb, director of the life sciences industry group at PRTM Management Consultants (Waltham, MA). “Not many can build a global organization by themselves,” he says. “You may even be better off selling to a bigger company with channel strength. Make sure you don't put in minefields that might hamper a future deal.”
SuturTek Inc. (North Chelmsford, MA) is an example of a company that found the right market opportunity and executed the right strategies. Its 360° Fascia Closure Device represented a major advance in the standard of care, and sales took off quickly. Indeed, the device won a Medical Design Excellence Award this year.
“Our technology is a replacement for hand suturing. We simply provided surgeons with a better method of doing a routine surgical task,” says Gerry Brecher, SuturTek's president and CEO. “The product is simple and easy to use, and as soon as surgeons see it, they say it's ‘cool'.” Surgeons were consulted during the design process so no unexpected use issues have emerged since the product hit the market. And a carefully managed development process translated to a carefully managed burn rate, so the firm didn't run out of money before getting its product to market.
In fact, there's only one thing SuturTek would do differently, Brecher says. “We learned that you need to hire a really ace manufacturing guy earlier rather than later. We got the manufacturing ready maybe a quarter too late because we didn't do that.”
Companies are more likely to succeed if they do that, says Dan Cole, general partner at Spray Venture Partners (Newton, MA). “Good companies think about manufacturing from day one,” he says. “That is particularly important if cost is a critical parameter. You must get your in-house or outside manufacturing up to the level of quality and reliability required as quickly as possible.”
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like