Has ResMed Hit Its Peak?
The sleep apnea device maker has gained significant market share during Philips' absence, but are those gains peaking?
August 8, 2023
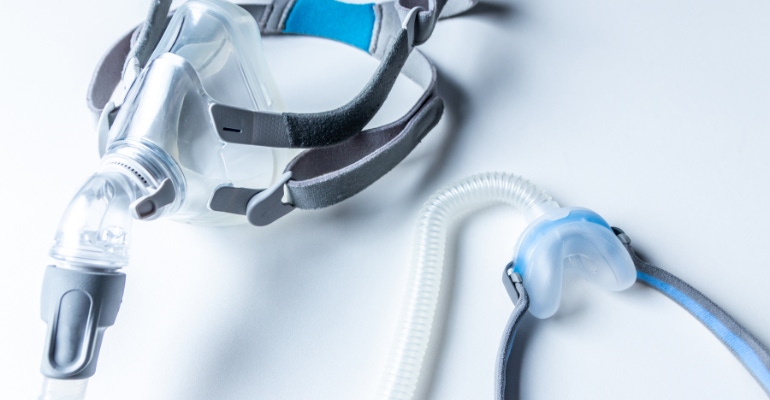
The big unknown in the sleep apnea world continues to be when Philips will re-enter the market. ResMed has more than risen to the challenge of meeting unprecedented demand in the competitor's absence, but now medtech analysts are wondering if ResMed's sleep apnea market share gains are peaking.
In the company's fiscal fourth quarter, ResMed missed analyst expectations on both revenue and earnings per share and saw its revenue growth slow to 23% from 32% on a constant currency basis compared to the previous fiscal quarter.
"It's unclear how much longer Philips will be out of the flow generator market," Mike Matson, a medtech analyst at Needham & Co., wrote in a recent report. "We think that it could reenter the market as soon as [the second half of 2023], or its FDA consent decree could keep it out of the market for several more years. If Philips does reenter the market in the nearer term, we think that [ResMed's] growth could slow as Philips recaptures some of its former market share."
The analyst noted that ResMed's previous supply chain challenges in terms of semiconductor shortages, logistics challenges, and inflationary pressures have nearly recovered.
During the company's recent earnings call, the management team reported that supply of ResMed's AirSense 10 product is now unconstrained, but there are still some lingering supply pressures impacting the AirSense 11. That said, the company is meeting all current sleep apnea market demand in the United States by allocating the AirSense 10 when the newer version is unavailable. Outside of the United States, ResMed's ability to meet customer demand is improving, the company noted during the call.
"Unconstrained availability of our market leading cloud connected flow generator platforms has enabled us to continue to offer access to 100% cloud connectable AirSense10 flow generated devices in all of our major global markets and beyond," CEO Mick Farrell said during the call, according to Seeking Alpha transcripts.
Farrell said the AirSense 11 platform is expected to gain further geographic regulatory approvals throughout the fiscal year and the sleep apnea market will see steadily increasing supply throughout the fiscal year 2024 and beyond.
"Although challenges within the post-COVID supply chain haven't completely been mitigated yet, we expect ongoing steady improvement in component and end product supply in the quarters ahead using a combination of AirSense 10 and AirSense 11 platforms," Farrell said.
The CEO emphasized that with combined availability of the unconstrained Air 10 platform, ResMed has enough devices to meet all the customer needs that it sees in major markets and globally.
ResMed CEO on sleep apnea market demand: We got this

Consistent with every ResMed earnings call since the beginning of Philips' massive recall on sleep apnea and other respiratory devices, Farrell had little choice but to comment on the competitor's ongoing recall.
Regardless of when Philips reenters the market, the company won't be coming back with the same market position in sleep apnea as it once had. And more to the point, the potential timing of a Philips come back doesn't seem to matter much to Farrell either way.
"Look, we have regional competitors in Europe that we are fighting with every day there," he said. "We have regional competitors in Asia that we're fighting with every day and regional competitors in the Americas that we're fighting with every day."
So, when Philips does come back, Farrell says it will have to start at position number four in terms of new patient setups. And in some countries, Philips never went away because the devices impacted by the company's foam degradation problems were never available in those regions, like in Spain.
"And in other markets in Europe where they've started to come back, we are competing and winning and maintaining share and growing share," he said. "I think the reputation here and the time to market is going to be a very slow progress for them country by country whether or not they get a consent decree in the largest geography."
The way ResMed looks at it, Farrell said, is that it is finally able to meet all the sleep apnea market demands between it and other regional players.
"So, it's almost irrelevant to us how and when [Philips returns] ... because we're able to take care of all the market growth," he said.
About the Author(s)
You May Also Like




