Establishing Substantial Equivalence of Reprocessed Single-Use Devices
Originally Published January 2001SINGLE-USE DEVICESIn light of the recent FDA enforcement decisions, companies submitting reprocessed SUDs for premarket review must pay careful consideration to scientific and regulatory considerations.David L. West, Timmie Topoleskie, and William MacFarland
January 1, 2001
Originally Published January 2001
SINGLE-USE DEVICES
David L. West, Timmie Topoleskie, and William MacFarland
 FDA has announced an intention to extend its enforcement of the premarket notification requirements of the Federal Food, Drug, & Cosmetic Act (FD&C Act) to reprocessors of single-use medical devices (SUDs). This is a major reversal of a policy established just two years ago.
FDA has announced an intention to extend its enforcement of the premarket notification requirements of the Federal Food, Drug, & Cosmetic Act (FD&C Act) to reprocessors of single-use medical devices (SUDs). This is a major reversal of a policy established just two years ago.
Under the new policy, published in Guidance for Industry and for FDA Staff: Enforcement Priorities for Single-Use Devices Reprocessed by Third Parties and Hospitals (August 2, 2000), a reprocessor will be required to submit data to FDA for each type of device it reprocesses. These data must be of sufficient quality and quantity for FDA to find that the device is safe and effective in reuse, or that it is substantially equivalent to another legally marketed device, such as the original disposable device. Any reprocessed device unable to meet this standard presumably will be denied approval or clearance for sale and thus will be removed from the market.
While FDA has said it will require premarket submissions for reprocessed devices, it has not elaborated on the substance of what must be included in them. The agency has not yet provided formal guidance to the reprocessing industry, or to its own reviewers, regarding specific data that will be required to clear or approve a reprocessed device. Reprocessing a device that was designed for only one use introduces many new technological characteristics into the device and raises significant questions not considered in previous device reviews. This article addresses some of those issues and proposes questions that should be asked in reviews of premarket submissions for all reprocessed SUDs. Through a discussion of design considerations and of data on reprocessed devices, it explores the effects of reprocessing on key areas of product integrity, sterilization, biocompatibility, shelf life and packaging, and labeling, and suggests the kinds of submission data needed to address those effects.
The purpose of this article is to suggest regulatory and scientific requirements for the marketing of used and subsequently reprocessed SUDs to ensure that each device is as safe and as effective as its new counterpart. It is to be hoped that this discussion will open a dialogue in the scientific and regulatory community that will result in effective FDA reviews of premarket submissions for these products—reviews that could prevent unsafe or ineffective devices from being used on patients.
EMERGENCE OF THE REPROCESSING ISSUE
Medical devices are articles defined by section 201(h) of the FD&C Act and are regulated under the authority of FDA (see insert below). Historically, disposable, so-called single-use medical devices have been understood to be devices conceived, designed, and manufactured for use in delivering care to one patient and then disposed of afterward.
Recent economic and social factors, however, exerting pressure to contain or reduce healthcare costs, have led to the common practice of reprocessing disposable medical devices for reuse on one or more additional patients. In some circumstances the reprocessing is performed by the user facility. But reprocessing of disposable devices has become so widespread that an industry of commercial reprocessors has emerged to collect used devices from user facilities and reprocess and resell them on a large scale. As a result, disposable medical devices are often reprocessed several times and used on multiple patients.
Despite its authority to regulate SUD reprocessing strictly, FDA has for years exercised regulatory discretion in ignoring reprocessors' failure to comply with certain elements of the FD&C Act. Reprocessed SUDs have not been subject to FDA clearance or approval for use in patients.
FDA premarket review is intended to investigate and uncover patient-safety issues through an examination of the device and its intended use. Only after successfully completing the premarket review process are complex medical devices permitted to be sold in interstate commerce.
In justifying its past decision to ignore reprocessor noncompliance with the premarket requirements, FDA in 1998 issued a call for data demonstrating safety risks posed by reprocessed disposables.1 Those data are now beginning to be revealed and their implications understood.
In light of growing concerns raised by the emerging data regarding the safety of reprocessing, FDA made its decision to increase regulation of reprocessed disposable medical devices through enforcement of all aspects of the FD&C Act, including submission of marketing applications to the agency for review. This involves requiring premarket notifications (510(k)s) or premarket approval (PMA) applications for some, but not all, reprocessed devices.
Because most medical devices are cleared through the 510(k) process, and because the vast majority of devices that are both disposable and economic candidates for reprocessing are not subject to PMA requirements, the body of this article will refer only to the 510(k) regulatory path. The discussion encompasses devices that traditionally have been regulated under the premarket notification process and also those that are, by regulation, exempt from premarket notification. However, the safety and effectiveness issues considered below in the context of devices regulated under section 510(k) of the FD&C Act would also apply to devices subject to PMA requirements.
PREMARKET NOTIFICATION AND REPROCESSED SUDS
In determining substantial equivalence (see insert), FDA examines whether the device in question is at least as safe and effective as the device to which it is being compared (the "predicate device"). FDA has established and disseminated many policies to ensure that these determinations of substantial equivalence serve the agency's public health mission, are scientifically sound, and collectively provide a predictable, orderly, and equitable regulatory process.
The most notable policy was issued in 1986 and established the 510(k) decision tree and rationale.2 The 510(k) decision tree involves determining substantial equivalence through a series of questions that probe, with increasing focus, the device's intended use and its technological characteristics (Figure 1). Requiring data from submitters of 510(k)s is the only way that FDA can determine, in a rigorous and systematic manner, whether the subject device is comparable to the predicate device. For legal and practical considerations to be satisfied, the subject device must be at least as safe and effective as the predicate device to be found substantially equivalent.
A 510(k) submission typically includes, in addition to various summaries and administrative information, the following elements:
Device classification information.
Labels and labeling, covering product identity, intended use, indications for use, and directions for use.
A description of the device, its technology, and its mode of operation.
Device design and performance specifications.
A comparison of the device's indications for use and technological characteristics to those of a predicate device.
Performance data, sometimes including clinical data.
Manufacturing information.
A 510(k) summary or statement.
To date, reprocessed devices have not been involved in the classification or 510(k) processes. Current device classifications (see insert) are based on the collective clinical experience of OEM devices. They were not intended to cover reprocessing or reprocessed devices. Because the reprocessing of disposable medical devices is a relatively recent phenomenon, and because the practice has not been satisfactorily described, reprocessed devices cannot be assumed to have contributed significantly to the collective clinical experience supporting FDA's classification of a specific device.
Moreover, the collective clinical experience supporting classifications of OEM devices cannot be assumed to attest to the safety or effectiveness of reprocessed devices. Reprocessed devices should not be deemed equivalent to their OEM counterparts without rigorous challenge in the context of a 510(k) review process designed to examine the technological characteristics that distinguish reprocessed devices from OEM devices.
New Technological Characteristics. A reprocessed product differs from the OEM version in having technological characteristics unique to reprocessing. These new technological characteristics arise because the device was designed by the OEM to be used only one time and is now being considered for use more than once.
In accordance with FDA's 510(k) guidance, the new characteristics must be taken into account in a 510(k) review. It is the agency's policy that a medical device may have new characteristics relative to the predicate device, but that accepted scientific methods must exist for assessing the effects of those characteristics on the device's performance, and that performance data must be available.
For FDA to apply its policies consistently and to ensure scientific integrity in the review of 510(k)s, it must make certain that the new technological characteristics are described in reprocessed-product 510(k)s, and that performance data assessing relevant safety and effectiveness issues are included. As with any 510(k), the submitter (here, the reprocessor) bears the burden of identifying validated methods for assessing the new technological characteristics and the safety and effectiveness issues associated with them.
To appreciate how reprocessing introduces new technological characteristics, consider the unavoidable differences between any new device and its reprocessed counterpart. The incoming "starting material" used by the reprocessor to manufacture the reprocessed device (that is, the used device) is of unknown quality, in contrast with the starting materials specified by the OEM.
Original-device manufacturers typically perform quality tests on starting materials. For example, a manufacturer of surgical staplers, upon receiving a lot of polycarbonate to be used in manufacturing the device handles, will randomly sample the polymer pellets for quality testing. Such testing, in order to be meaningful, is necessarily destructive. If the samples pass the quality test, polymer pellets from that lot are then used to manufacture the stapler handles. The OEM can use random samples to characterize an entire lot of material because that material is made using validated processes that result in a homogeneous product.
Reprocessors, however, do not obtain a homogeneous lot of raw material and thus cannot perform traditional material testing. Testing performed by the reprocessor is limited to that which will not alter or destroy the device. Such testing is naturally incomplete because it cannot uncover latent defects that could lead to device failure. Even testing every single device in this way will not provide the assurance of material integrity possible with random destructive material testing.
Moreover, the reprocessor's incoming material has a use history that may not be known with certainty and will be different for each device. Yet the history of each device is central to its quality as a reprocessed product.
The new technological characteristics of the reprocessed device raise important questions in the following areas, each of which must be addressed by proper performance data.
Product physical integrity.
Sterilization and cleaning.
Biocompatibility.
Shelf life and package integrity.
Labeling.
The answers are critical to a finding that the reprocessed device is substantially equivalent to—that is, as safe and effective as—the predicate device. For original devices, these questions are typically addressed by either design verification or validation as required by FDA's quality system regulation.
In view of the substantial changes in an SUD brought about by reprocessing, and of the existing evidence of reprocessed-device failures, reprocessed devices cannot be presumed equivalent to their OEM counterparts. The reprocessor must address this issue prior to patient exposure, in a premarket submission. The existence of substantial data showing serious product defects exhibited by reused devices found on hospital shelves suggests the inadequacy of the FDA inspection authority to control these safety issues effectively.
Performance Data. In reviewing performance data, FDA reviewers should keep in mind that the new technological characteristics involve both product design issues and process issues. This means that performance data for reprocessed-device 510(k)s will normally include both design verification testing and process design verification testing.
Design verification and process design verification questions that should be asked in reviewing reprocessed-SUD performance data to address new technological characteristics introduce each of the following discussions on the areas of concern identified above. Each discussion considers some of the performance problems that have been reported, as well as other problems that could arise.
PRODUCT PHYSICAL INTEGRITY
New technological characteristics introduced by reprocessing raise questions about product physical integrity such as the following:
Have performance specifications been established that translate into adequate clinical performance? How will the device be tested for conformity to those specifications?
How are nonobvious OEM design changes detected and accounted for?
What is the extent of material and mechanical degradation following use, cleaning, and sterilization?
How has reliability for each subsequent reuse been addressed?
What are the potential failure modes of the reprocessed device?
How is the test procedure designed to measure failure?
How will the number of reuses of a product be tracked?
Regarding the last question, FDA has made clear its intent to approve reprocessed SUDs for just one additional use despite multiple-use practices already firmly established by reprocessors. Failing to require data to support the maximum allowable number of reuses is inconsistent with FDA's regulation of other multiple-use devices.
Device Use History. Whereas an OEM product has no use history, a reprocessed product may have been used once or many times, and the nonhomogeneity of that use history must be taken into consideration. The unique use history of a reprocessed device could have a significant impact on future performance. In fact, reuse of individual units of the same model of a device, all reprocessed in the same way, could lead to completely different outcomes determined by conditions of prior use, such as the anatomy of the patient on whom the device was first used.
For instance, a surgical needle previously used in scar tissue may exhibit a markedly different failure profile than a needle that has merely passed through normal tissue. Future performance and possibility of failure of these two seemingly identical needles is likely to be different. A 510(k) for such a reprocessed device would need to include performance data that assess the effects of this new technological characteristic (varying failure profile) and demonstrate equivalence to the OEM device.
Similarly, the atherosclerotic plaque that blocks the coronary arteries of angioplasty patients may differ in hardness and pliability from patient to patient. The same type of percutaneous transluminal coronary angioplasty (PTCA) catheter may thus be subjected to very different inflation pressures, material interactions, and other conditions in different patients. Such differences will affect the future distensibility of the balloon components of PTCA catheters employed to reestablish normal flow in the artery. Such balloons are designed to inflate to preset diameters. This performance feature degrades as the balloon is repeatedly stressed with reuse, and may be compromised to an unknown degree by aspects of the anatomy of patients on which it was previously used.
OEM product performance testing is concerned with initial failure. By contrast, performance testing for reprocessed products must take device exhaustion, or wear-out, into consideration. This means that, in addition to assuring that the reprocessed product meets functional specifications, the reprocessed-device 510(k) must characterize time to failure or number of uses to failure.
Because its use history is unknown and destructive device testing is not feasible, it may be impossible to inspect or test a product to determine whether it is at or near the wear-out stage of use. The 510(k) for such devices must identify alternative methods for detecting and, more importantly, predicting such wear-out. Otherwise, such failures will only be detected in use, possibly resulting in patient injury or medical error.
Material Effects of Reprocessing. In addition to prior use, reprocessing itself may alter key material and mechanical properties of the device. For example, cleaning and sterilization may introduce new failure modes to the device or accelerate the wear rate of a reprocessed product.
Some stainless steels, including mar-tensitic alloys of the 400 series, designed for maximum hardness, are used in the manufacture of disposable scalpel blades and knives. The material mechanisms that impart high hardness to these stainless steels render them especially susceptible to corrosion. Small scratches left from grinding, sharpening, or scrubbing may be readily attacked by certain cleaning and sterilization chemicals, including peracetic acid, which can lead to corrosion. Such corrosion can in turn lead to catastrophic failure of the instrument on subsequent use.
For reprocessed stainless-steel devices, then, it is imperative that the 510(k) characterize these new technological characteristics. In addition to identifying the type of stainless steel used to manufacture the device, the reprocessor would need to characterize the likely failure points on the device and the average number of cleaning and sterilization cycles the device could undergo prior to such failure.
Many studies focusing on reprocessed SUDs have reported malfunctioning or out-of-specification devices. These studies are useful in identifying changes introduced by reprocessing that must be accounted for in the 510(k).
In an FDA study of the effects of cleaning and reuse on device materials, both used and new, purposely contaminated PTCA balloon catheters were cleaned, packaged, and EtO sterilized before being analyzed. Results demonstrated that the guidewire tubes of a number of reprocessed balloons were curled up inside the balloon as a result of overinflation, while other balloons shrank due to reprocessing and EtO sterilization. Analysis of the unused balloons subjected to cleaning revealed changes in balloon diameter as much as 10% outside of the manufacturer's specification. Results also showed that reprocessed balloons were stickier than new balloons.3
One study demonstrated functional failures specific to balloon catheters. These included poor trackability, probably due to the unfolded condition of the balloon as it was removed from the package; inability of balloons to be prepared in accordance with instructions for use; curved inner bodies and S-shaped inner bodies in the balloon upon inflation for testing; and failure to withstand an average burst pressure of 21 atm.4
In that same study, functional failures involving diagnostic and guiding catheters included failure of reprocessed diagnostic catheters to perform as well as new catheters in two types of torque testing and signs of material degradation in the guiding catheters following tensile overload tests. All of the guiding catheters and two-thirds of the diagnostic catheters displayed out-of-tolerance shape conformance. One-third of the diagnostic catheters had outside-diameter measurements above their preestablished specifications and top inner diameters that were below preestablished specifications. Failure to withstand five injections to the pressure rating indicated on their hubs characterized one-fifth of the diagnostic catheters.
A corporate study subjected 27 reprocessed cardiac catheters to functional testing. All balloon catheters failed to perform adequately in trackability and balloon preparation tests. In addition, 9% of diagnostic and guiding catheters failed tests for shape conformance, and up to 56% of all catheters did not pass some aspect of the visual inspection, exhibiting such faults as flaking of the exit marker, kinks, bends, a sliced outer body and strain relief, or an open fuse.5
Clearly, resterilization and the use history of reprocessed catheters contribute many new technological characteristics to these devices, which must be defined and evaluated in 510(k)s as part of the bid for a conclusion of substantial equivalence. While much of the available data on reprocessed SUDs concerns cardiac catheters, several other device types have also been studied, including orthopedic devices.
In one study, investigators collected reprocessed devices from area hospitals to assess whether they still conformed to the specifications of a new device.6 Devices studied included cutting accessories, which are precision instruments designed to rotate at up to 100,000 rpm while delivering clean cuts. Results of the investigation demonstrated that attempts to resharpen a used device removed essential material, which depleted and destabilized the device, making it unreliable. The resharpening of such devices has produced misdimensioned cutting accessories and flawed instruments that could result in longer surgeries and poor surgical outcomes. Of the 213 devices analyzed, 81 (38%) had flaws in their integrity. Of these 81 devices, 23 had worn, damaged flutes.
In this case, a lack of design understanding on the part of the reprocessor seems to have led to inappropriate reprocessing. A 510(k) for such a reprocessed device would need to include data that characterize the resharpened surface and evaluate the device's ability to operate as intended. Such data would be necessary to support the claim of substantial equivalence.
A similar report revealed that an ultrasonic device blade had not been uniformly sharpened on reprocessing. Ultrasonic cutters must vibrate at a particular frequency in order to achieve their dual cutting and coagulation effect. Imperfect shaping, scratching, or other surface damage to the blade of such a device will cause it to vibrate improperly. As a result, the device may not activate in midprocedure; or it may cut but not coagulate, resulting in internal bleeding; or the blade may fracture during use, possibly depositing fragments in the patient. So that such negative outcomes may be avoided, the reprocessed-device 510(k) must demonstrate the reprocessor's clear understanding of the device's key design features and functionality.
This example demonstrates the importance of having FDA reviewers who are fully conversant with the safety trade-offs inherent in reprocessed devices. How FDA will actually decide which trade-offs are acceptable, in light of its requirement that all 510(k) devices must be at least as safe as their predicate devices, has not been addressed in the reuse guidance document.
In addition to design considerations, a reprocessed-device 510(k) must identify all the materials used in the device and account for the effects of reprocessing on each one. Reprocessing can significantly affect the integrity of a device. Postprocess testing must ensure that processing steps, including sterilization, do not adversely affect a device's material or mechanical properties. Final testing conducted before sterilization is insufficient to provide such assurance.
Damage to metals, polymers, and adhesives found in devices taken from hospital shelves demonstrates the ineffectiveness of reprocessed-device testing prior to sterilization as a control for these types of safety issues. This is not surprising from a regulatory perspective. It confirms the longstanding FDA policy that product quality and reliability cannot be achieved through device testing. These attributes must be designed into medical devices, and devices must be manufactured with validated processes to ensure reproducibility.
For instance, aluminum used to manufacture disposable intubation stylets may develop subcritical damage that leads to an undetectable flaw and then device failure. A serious injury to a surgical patient was reportedly brought about by the use of a reprocessed aluminum intubation stylet.7 During a difficult intubation, a 10-cm section of the disposable stylet broke off in the patient's esophagus. It was not detected until several weeks later when the patient reported acute stomach pains, which were traced to the stylet fragment having perforated the duodenum.
This incident underscores the need to have the performance characteristics of a device designed in. It also reinforces the importance of understanding how many times a device can be reused before it will fail, and of the need to provide an adequate safety margin for patients.
Testing a device before release merely demonstrates that it functioned when tested. It does not provide assurance that the device will work the next time it is used on a patient.
In the case of devices manufactured from polymeric materials, the 510(k) for a reprocessed SUD must identify the various polymers used in the construction just as the 510(k) for the OEM device must. Further, to address the new technological characteristics of these reused devices, the reprocessor needs to characterize the susceptibility of each polymer to various reprocessing changes along with the effect of such changes on both material integrity and device mechanics. Absorption of cleaning fluids, for instance, can have a plasticizing effect on some polymers and alter their mechanical properties.
In one in-house study, a reprocessed single-use trocar developed both a complex crack in the housing near the stopcock and a chip in the leading beveled edge of the trocar sleeve after reprocessing. The disinfecting solutions used in reprocessing weakened the plastic and caused it to crack at stress points.8 Cracks at stress points can cause a trocar to break during reuse, resulting in plastic shards in the patient.
Many single-use devices incorporate long, narrow, polymer tube components. Such components can easily bend and kink and thereby produce crazing, an alignment of polymer molecules that changes the fundamental mechanical properties of the polymer and increases the likelihood of device failure. Crazing causes the distinct white line that often forms at the point where plastic products are bent. This line signals a change in the polymeric properties and marks the most likely site of failure.
Model-by-model process design verification is essential in 510(k) review of reprocessed devices in order to ensure that devices at or near the point of critical failure are identified. In fact, FDA's Office of Science and Technology (OST) has stated, in response to results of testing in agency laboratories, that only model-by-model evaluations of reprocessed SUDs would be acceptable.9 The way that different polymeric materials used to manufacture similar devices may demonstrate markedly different degradation profiles over time is charted in Figure 2. Mechanical degradation can be related to fatigue, wear, loss of tensile strength, crazing, or another precursor of failure.
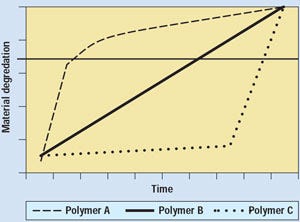 Figure 2. Possible polymer failure profiles, showing why it is essential to identify materials of construction in reprocessed-device 510(k)s.
Figure 2. Possible polymer failure profiles, showing why it is essential to identify materials of construction in reprocessed-device 510(k)s.
The effect of multiple uses is not addressed in the 510(k) for a new single-use device. Therefore, the reprocessed-device 510(k) must identify the types of polymers used in its manufacture, along with their degradation profiles, in order to appropriately identify predictors of failure. Devices made from polymers A or C in the figure may fail catastrophically with little warning, the polymer-A device perhaps after first use. The polymer-C device may be reprocessible, but it will likely fail without warning. Devices made of polymer B may act more predictably. For devices in which signs of wear or degradation are not evident, accurate tracking of use history is essential in order to minimize the possibility of patient injury.
Most single-use medical devices are made up of several different polymers or metals. The abutment of different materials generates additional reprocessing questions. Adhesives used to join materials can
You May Also Like


