Why Metal Finishing Matters in the Medtech Industry
April 30, 2015
Metal finishing removes burrs, improves corrosion resistance, increases part longevity, and creates an ultraclean, sanitary finish.
Bob Michaels
|
Metal finishing processes are crucial for manufacturing titanium screws. |
The medical device industry faces multiple challenges when it comes to manufacturing reliable, hygienic, and compliant parts and devices, remarks Tom Glass, president of Able Electropolishing (Chicago). Thus, medical device manufacturers should perform a range of finishing operations to ensure optimal performance. Here's what Glass has to say about the importance of metal finishing in the medical device industry.
* * * * *
How Can I Meet Biocompatibility Challenges? |
Many industries utilize finished metal parts in their processes, machinery, or end products, and the medical device industry is no exception. In the medtech world, metal finishing is an important final step in the manufacture of devices and components.
Take the question of biocompatibility, for example. Composed of metal components, orthopedic devices such as knee, hip, shoulder, and elbow implants and cardiovascular implants such as defibrillators, pacemakers, and artificial heart valves confront biocompatibility challenges. Manufacturers can address these challenges by employing a variety of metal finishing operations with the goal of removing surface imperfections.
What's in It |
|
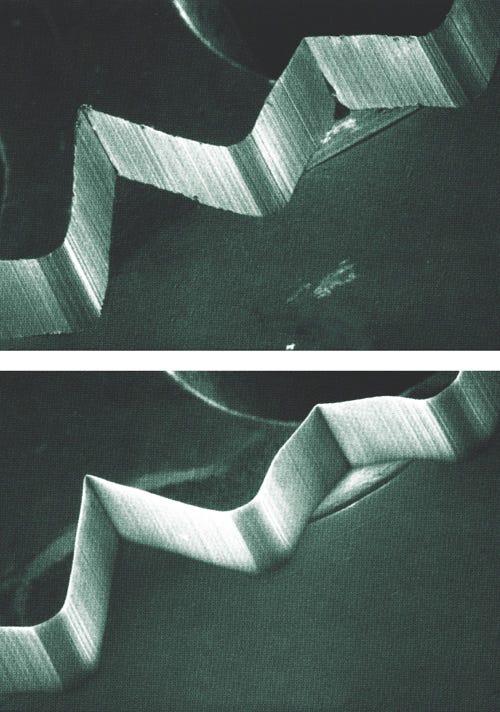
SEM images of metal pieces before (top) and after (bottom) undergoing a metal finishing step. |
Whether you manufacture pacemakers or hip implants, metal finishing addresses a variety of part imperfections acquired through the manufacturing process. Quality metal finishing offers a host of advantages, including the removal of burrs, improved corrosion resistance, increased part longevity, and the creation of an ultraclean, sanitary finish that inhibits bacterial formation on the part surface. Whether the manufacturer wishes to remove heat tint from welded areas or to eliminate microcracks that can result in premature part failure, metal finishing helps ensure device performance over time. Metal finishing treatments can also address microfinishing and sizing concerns.
In addition to improving part quality and performance, medical device manufacturers must meet stringent industry standards and ensure reliability. Quality processes and quality management systems such as those set forth by ISO 13485 and ISO 9001 enable medical device manufacturers to meet these demands. But in addition to allowing manufacturers to meet industry standards, these certifications ensure consistent results from lot to lot and from part to part. That's where metal finishing operations come in.
What's in It |
After undergoing implant surgery, patients can suffer from a range of issues, including infections, implant rejection, or prolonged recovery times. By employing a variety of metal finishing processes, medical device manufacturers can improve the quality and performance of their products, benefiting patients as well as their bottom line.
When an implant is rejected, the patient must undergo revision procedures and additional treatments to fight infection or other side effects. While prolonging recovery times, extended treatments can also deteriorate a patient's overall health. In addition to increased medical costs, long hospital stays often cause the patient to suffer from muscle atrophy, increased anxiety, and emotional distress.
Implants that have undergone advanced metal finishing treatments are less likely to be rejected and less likely to cause surgery-related health issues than unfinished implants, improving patients' overall quality of health.
What Metal Finishing Process Are Out There? |
Most of the metal finishing processes available to industry as a whole are also available to the medical device industry and manufacturers of surgical instruments. Blasting, tumbling, plating, passivation, and electropolishing are just a few of the metal finishing processes that can improve medical device manufacturing applications.
For example, stainless-steel parts can be passivated in a nitric or citric acid bath to remove free iron and foreign materials from their surfaces. However, while passivation alone can clean the part and effectively remove free iron, it cannot produce a microfinished or a sufficiently corrosion-resistant surface, rendering it inadequate for certain medical device applications.
Microfinishing and corrosion resistance are best achieved using electropolishing, a process by which parts are submerged in a chemical bath and treated with electricity. Although many manufacturers prefer the use of passivation to finish their metal components, this method cannot achieve the same high-quality finish as electropolishing can.
Electropolishing is unique in that it can achieve multiple benefits. It can eliminate surface contaminants, brighten and polish metal, remove burrs, eradicate heat tint and oxide scale, improve microfinishes, and make parts 30 times more corrosion resistant than passivation can. Consequently, electropolishing is the best metal finishing option for medical device applications.
Why Is Process Improvement Important? |
|
Different types of metal parts can undergo a range of metal finishing operations. |
Advances in the medical industry such as the use of titanium and nitinol require improved finishing processes. For example, titanium is used widely in the medical device industry because it is lightweight, improves tissue growth, and has a lower rejection rate than other materials. Nevertheless, while titanium alloys are superior to other materials in medical device applications, they must still undergo quality finishing processes, making it incumbent on OEMs to collaborate with metal finishing service providers to produce the safest products possible.
About the Author(s)
You May Also Like