Thermoplastic Silicone-Urethane Copolymers: A New Class of Biomedical Elastomers
As part of the ongoing quest for more effective biomaterials, novel families of copolymers are combining the beneficial properties of silicones and polyurethanes.
April 1, 2000
Only a limited number of elastomers have the demonstrated biostability and biocompatibility to serve reliably in long-term medical implants. For more than 30 years, two biomaterials have been used extensively in implantable devices: cross-linked silicone rubber and thermoplastic polyurethane (TPU). For products that combine the need for physical endurance with long implant times, TPUs—and the closely related, solvent-cast, segmented polyurethanes (SPUs)—have often been specified. Currently, new indications and even longer implant times for critical devices are mandating continual improvements in the stability, toughness, and biocompatibility of elastomeric biomaterials. These polymers are candidates for use in applications as diverse as vascular grafts, cardiac-assist devices, prosthetic heart valves, joint repair/replacement products, urological implants, electrical lead insulation, and drug-delivery catheters. At the other end of the medical device spectrum are short-term implants and certain disposable products. Here, new polymers are being sought that can replace natural rubber latex, a material whose desirable properties of good "memory" and low modulus are counterbalanced by a tendency to provoke protein-induced allergic reactions.
 Molten silicone-urethane copolymer material emerging from continuous reactor onto moving, Teflon-coated stainless-steel belt.
Molten silicone-urethane copolymer material emerging from continuous reactor onto moving, Teflon-coated stainless-steel belt.
This article describes a new class of biomaterials—thermoplastic silicone-polyurethane copolymers—that in combining the benefits of silicone rubber and TPU offer the potential for use both in long-term implants and as latex-replacement materials for disposables and other devices. Following a brief discussion of the constituent materials and of earlier efforts to modify polyurethanes using silicone, an outline of the process used to design these new materials and a review of their characteristics are presented.
SILICONES
Long known to be biostable and biocompatible in most implants, silicones also frequently have the low hardness and low modulus that are useful in many device applications. Conventional silicone elastomers can have fairly high ultimate elongations, but only low-to-moderate tensile strengths. Consequently, as measured by the area under their stress-strain curves, the toughness of most biomedical silicone elastomers is not particularly high (Figure 1). In addition, poor cut-growth propagation (for example, when nicked by a scalpel) and the universal need for reinforcing fillers can also be considered minor disadvantages in the use of silicones as biomaterials.
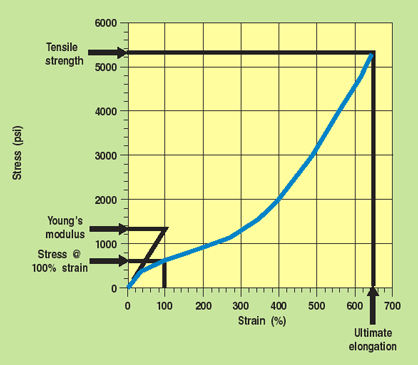
Figure 1. Typical stress-strain curve for an elastomer showing how tensile properties are derived from the plot. The area under the curve is the sample toughness.
One of the least attractive properties of conventional silicone elastomers in device manufacturing is that the materials require covalent cross-linking to develop useful properties. Linear or branched silicone (polydimethylsiloxane (PSX)) homopolymers are viscous liquids or millable gums at room temperature. Fabrication of device components must include, or be followed by, cross-linking to form chemical bonds among adjacent polymer chains. The infinite network thus formed gives the polymer its rubber elasticity and characteristic physical-mechanical properties.
Cross-linking of extrudable and moldable silicone stock is usually done via peroxide-generated free radicals adding to vinyl groups incorporated along the polymer backbone, or, increasingly, by the platinum-catalyzed addition of silane (-Si-H) to terminal vinyl groups in so-called LIM systems. Certain low-strength (RTV) silicone adhesives vulcanize at room temperature by condensation reactions, eliminating an acid or alcohol to generate -Si-OH or silanols, followed by the elimination of water as silanols condense to form -Si-O-Si- (siloxane) bonds and create a three-dimensional network.
Regardless of how the cross-linking or vulcanization is effected, the resulting thermoset silicone cannot be redissolved or remelted. This reduces the number of postfabrication operations that can be used in device manufacturing with these silicones, relative to those possible with thermoplastic biomaterials. For instance, thermal forming, tipping, and tapering; radio-frequency welding; heat sealing; and solvent bonding are all useful postfabrication methods that are essentially unavailable when building devices from conventional silicone elastomers.
THERMOPLASTIC POLYURETHANES
In contrast to cross-linked silicone rubbers, many polyurethane elastomers are thermoplastic in nature. That is, they can be processed by methods that involve melting or dissolving the polymer to reshape it. The molecular structure of a typical biomedical TPU consists of alternating high-melting "hard" urethane segments and liquidlike "soft" segments. Hard segments are almost always the reaction product of an aromatic or aliphatic diisocyanate and a low-molecular-weight, chain-extending dialcohol or diol. In the TPUs used as biomaterials, soft segments are usually built from (polyether or polycarbonate) polyols with terminal hydroxyl (-OH) groups.
 Automated dipping of intraaortic balloons from solvent-cast, first-generation silicone urethane (circa 1976).
Automated dipping of intraaortic balloons from solvent-cast, first-generation silicone urethane (circa 1976).
The reaction of isocyanate (-NCO) and hydroxyl creates a urethane group, while the reaction between isocyanates and existing urethane groups will form allophanate groups that can produce minor amounts of covalent cross-linking in TPUs. When a TPU is heated, the hydrogen-bonded hard segments and any allophanate cross-links—both of which hold the polymer together at its use temperature—dissociate to allow the polymer to liquify and flow. Dissolution in a polar solvent can also disrupt the hydrogen bonds that hold together the hard segments on adjacent chains. Once these virtual cross-links are broken, the polymer can be fabricated into useful shapes. Upon cooling or solvent evaporation, the hard segments de-mix from the soft segments to reassociate by hydrogen bonding. This restores the original mechanical properties of the polyurethane elastomer.
Conventional polyether and polycarbonate TPUs generally have excellent physical properties, combining high elongation and high tensile strength to form tough, albeit fairly high-modulus, elastomers. Whereas natural rubber latex may have an initial modulus of a few hundred pounds per square inch, an 80A aromatic polyetherurethane might have a modulus of >2000 psi, making it considerably less compliant. Aromatic polyether TPUs, on the other hand, can have excellent flex life, a tensile strength of >5000 psi (34 MPa), and ultimate elongations of >700%. They are often used for continuously flexing, chronic implants such as ventricular-assist devices, intraaortic balloons, and artificial heart components.
The two most important diisocyanates used in biomedical TPUs are aromatic diphenylmethane diisocyanate (MDI) and its hydrogenated analog (HMDI, or H12MDI). TPUs with hard segments made from MDI typically have superior physical properties and chemical resistance relative to analagous TPUs made from HMDI, especially when compared at body temperature in an aqueous environment typical of blood or tissue. Varying the total hard segment content of a TPU during synthesis can produce a whole family of polymers of related chemistry but with a wide range of hardness, modulus, tensile-strength properties, and elongation. In device applications, the use of TPUs of different hardness values within a single family provides considerable versatility in design and manufacturing.
SILICONE-MODIFIED POLYURETHANES
Silicones preceded polyurethanes as biomaterials by several years. Even as the first polyurethanes were being used in medical devices, some investigators recognized the possible advantages of combining silicone and polyurethane in a single biomaterial.1,2 Since approximately 1970, the approaches have included coatings, blends, interpenetrating networks, surface-modifying additives in urethane base polymers, and, most recently, high-strength "structural" copolymers of silicone and polyurethane.3,4,5
In these newer copolymers, the silicone may be present along the polymer backbone and/or in the form of covalently bonded end groups or grafts.6 Because of the surface activity of silicones in organic base polymers (even when compolymerized), it is relatively easy to obtain silicone-like surface properties on polyurethanes at very low silicone content.7 This is particularly true when the silicone is present in the copolymer as mobile end groups that can "self-assemble" at the copolymer surface.8 A bigger challenge has been to obtain some of the other desirable silicone properties—for example, biostability or low modulus—while preserving such excellent bulk properties of polyurethanes as their optical clarity, thermoplastic processability, and toughness.
In 1970, the solvent-cast silicone-polyurethane Avcothane-51 was introduced by Avco Everett Research Laboratory (Everett, MA) as the material of construction for the first clinical intraaortic balloon. (Avcothane-51 has since been renamed Cardiothane-51 and continues to be used in what is now the Arrow intraaortic balloon.)1,2 This combination of silicone and polyurethane was first proposed to improve the thromboresistance of early cardiac-assist devices, which at the time were plagued by gross thrombogenicity. Cardiothane-51 has since been reported to have excellent thromboresistance, flex life, abrasion resistance, and biostability. As the silicone-urethane system that has had the most clinical use in critical medical devices, it is best characterized as a hybrid in which a silicone-urethane copolymer formed in situ stabilizes a blend of a silicone homopolymer in polyurethane.2 After the organic solvent evaporates, the homopolymer cross-links upon exposure to atmospheric moisture by the RTV reaction described above.
SILICONE-URETHANE COPOLYMERS
Despite excellent in vivo results, the Avcothane/Cardiothane system has several disadvantages that have limited its use as a biomaterial. These include the need for solution processing, the tendency of the solution to prematurely react with water and coagulate, and the dependence of polymer surface properties on reaction and fabrication conditions. In addition, the polyblend nature of Cardiothane and the thermodynamic incompatibility of silicone with most polyurethane reactants leads to the formation of silicone microdomains large enough to scatter light. This causes Cardiothane and other hybrid systems to be opaque or translucent to visible light, depending on the actual size of the domains.
Since the introduction of Avcothane-51, many investigators have sought to develop high-strength, thermoplastic silicone-polyurethane (TSPU) copolymers. The prospect of combining the biocompatibility and bio-stability of conventional silicone elastomers with the processability and toughness of TPUs is an attractive approach to what would appear to be a nearly ideal biomaterial. For instance, it has been reported that silicone acts synergistically with both polycarbonate- and polyether-based poly-urethanes to improve in vivo and in vitro stability—as, for example, when covalently bonded to the urethane during synthesis. Several copolymers have shown increased resistance to metal ion–induced oxidation and environmental stress cracking, both of which have been identified as modes of polyurethane failure in applications such as some early polyurethane pacemaker leads. In polycarbonate-based polyurethanes, silicone copolymerization has been shown to reduce hydrolytic degradation of the carbonate linkage (Figure 2), whereas in polyether urethanes, the covalently bonded silicone seems to protect the polyether soft segment from oxidative degradation in vivo.9

Figure 2. Scanning electron micrographs (200x) of explants of low-hardness (65A) segmented polycarbonate-urethanes after 12-month intramuscular implantation in rabbits showing enhanced biostability via silicone copolymerization: (left) neat polycarbonate-urethane with environmental stress cracking; (right) silicone-polycarbonate-urethane copolymer with no stress cracking.
Despite these encouraging results, a number of factors have delayed the introduction of a useful family of TSPU biomaterials. Problems include the lack of solubility of PSX in most urethane coreactants and the resulting difficulty this creates in the synthesis reaction. Additional problems are the lack of low-cost, urethane-grade silicone polyols, and the absence of synthetic methods that could consistently yield high-molecular-weight copolymers without using organic solvents.
Over the past several years, work has been ongoing to develop new silicone-polyurethane copolymers and the precursors and manufacturing processes needed to synthesize them (Figure 3). Copolymer synthesis has been performed by combining two previously reported methods: incorporation of silicone into the polymer backbone together with organic (nonsilicone) soft segments, and the use of surface-modifying end groups to terminate the copolymer chains.4,8,10 Through bulk synthesis (without solvents), four families of high-strength thermoplastic elastomers have been prepared that differ in hard-segment chemistry and content and in the organic soft segments used in combination with silicone polyols. In one class, the organic soft block is polytetramethyleneoxide (PTMO); in the other, it is an aliphatic polycarbonate (PC) soft segment used together with PSX.
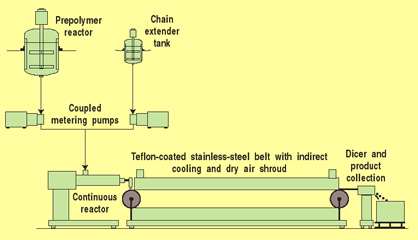
Figure 3. Continuous polymerization process used in the manufacture of thermoplastic silicone-urethane copolymers.
Soft-segment molecular weights vary depending on the hard-segment content. PTMO- and PC-based families with both aromatic and aliphatic hard segments have also been synthesized, using diol chain extenders. Copolymers have been made in which silicone content ranges from <1% up to the total soft-segment content of the polymer, which can be from 20 to 65% depending on copolymer hardness.
Many of these silicone-urethane copolymers demonstrate previously unavailable combinations of properties. For instance, aromatic silicone-polyetherurethanes have a higher modulus at a given Shore hardness than do conventional poly-etherurethanes: the higher the silicone content, the higher the modulus. The aliphatic silicone-polyetherurethanes, on the other hand, have a very low modulus and high ultimate elongation, which is typical of silicone homopolymers or even natural rubber. In both the PTMO and PC families, certain polymers have tensile strengths three to five times higher than those of conventional silicone biomaterials.
All four material families present opportunities to optimize formulations for specific applications. Although less than 1% silicone can give silicone-like surface properties, higher silicone content is required to obtain silicone-like bulk properties such as biostability, permeability, or reduced water absorption.11 Not all the bulk properties of silicone are desirable in every application. (Its low tensile strength is seldom desirable in any application.) Thus, the more silicone that is used to replace the organic soft segment, the lower the tensile strength becomes (Figures 4 and 5). In addition, higher silicone content increases the ultimate raw materials cost of the polymers. Clearly, there is an incentive to determine the minimum silicone concentration that will produce the desired balance of properties.
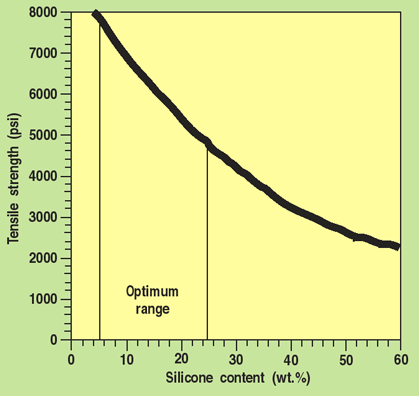
Figure 4. The relationship between tensile strength and silicone content in two families of aromatic thermoplastic silicone-urethane copolymers. For many biomedical applications 5 to 25% by weight appears to be an optimum. If high permeability is required—e.g., in membranes—a higher silicone content may be used. Note that even when silicone content is as high as 60 wt% the thermoplastics can have tensile strength exceeding most conventional silicone elastomers at 1000 to 2000 psi.
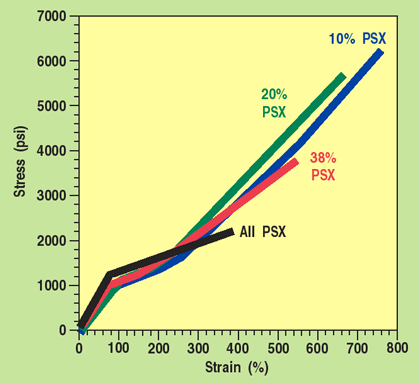
Figure 5. Stress-strain curves of four aromatic silicone-polyether-urethane copolymers showing the effect of silicone content on tensile strength.
As mentioned above, certain of the aliphatic polyether-silicone copolymers appear to have an unusual combination of toughness (high tensile strength and elongation) and very low initial modulus. This makes them attractive as high-performance substitutes for conventional cross-linked silicone elastomers. Depending on silicone content, modulus can be as low as that of natural rubber latex, with tensile strength equal or greater than that of natural rubber. Increased modulus in the aromatic series is believed to be due to enhanced hard-segment/soft-segment phase separation resulting from the very low solubility parameter of silicone. The converse may be true in the case of aliphatic hard segments, which appear to be more compatible with soft segments containing silicone.
In both aromatic and aliphatic silicone-polyurethane copolymers, optical transparency is possible over a wide range of total silicone concentration: from <1 to >65% by weight. This is quite different from typical optical properties of hybrid or interpenetrating network systems, in which the gross phase separation of silicone often results in opacity to visible light.
Many of the synergistic benefits of silicone are obtained at low to moderate silicone concentrations, which can preserve the mechanical strength of the parent polyurethane. Furthermore, if the inherently high gas and vapor permeability of PSX is to be avoided in specific applications, it is desirable to keep the total PSX concentration low. In the silicone-urethane copolymers reported here, the silicone concentration is completely variable depending on the end use. Over the full PSX concentration range, optical clarity is possible when desirable—for example, in blood tubing, catheters, and cannulae.
The extrusion of the new additive-free copolymers on both single-screw (24/1 L/D) and twin-screw extruders has given excellent surface finish and low gel counts relative to conventional thermoplastic polyurethanes (for example, Pellethane 2363 80A and Tecoflex 80A polyether-urethanes, and Bionate 80A polycarbonate-urethane). The polymers accept fillers well and are heat-sealable and easily postformed. They are also soluble in organic solvents, allowing for solution processing and solvent bonding.
CONCLUSION
Novel families of silicone-urethane copolymers appear to provide a combination of biostability, advantageous physical properties, and processability that has not previously been available in conventional polyurethane biomaterials. They are, therefore, promising candidates for use in the fabrication of a wide range of medical products. Pending the completion of biostability testing, these materials may be considered for use in chronically implanted devices and prostheses, including leads, grafts, balloons, shunts, cardiac-assist devices, urological implants, and stents.
REFERENCES
E Nyilas, Polysiloxane-polyurethane block copolymers, US Pat. 3,562,352, 1971.
RS Ward and E Nyilas, "Production of Biomedical Polymers I: Silicone/Urethane Synergy in Avcothane Elastomers," Organometallic Polymers (1978), 219222.
RS Ward et al., "Use of Surface-Modifying Additives in the Development of a New Biomedical Polyurethaneurea," Polyurethanes in Biomedical Engineering, eds. H Planck, G Egbers, and I Syre (Amsterdam: Elsevier Science Publishers B.V., 1984).
RS Ward, Polymer systems suitable for blood-contacting surfaces of a biomedical device and methods for forming, US Pat. 4,675,361, 1987.
P Litwak et al., "Development of a Small-Diameter, Compliant, Vascular Prosthesis," in Proceedings of the UCLA Symposium on Molecular and Cell Biology (Lake Tahoe, CA: University of California, 1988).
RS Ward et al., Copolymers and nonporous, semipermeable membrane thereof and its use for permeating molecules of predetermined molecular weight range, US Pat. 5,428,123, 1995.
RS Ward, "Surface Modification Prior to Surface Formation: Control of Polymer Surface Properties via Bulk Composition," Medical Plastics and Biomaterials, 2 (Spring 1995): 34–41.
RS Ward et al., Surface-modifying end groups for biomedical polymers, US Pat. 5,589,563, 1996.
AB Mathur et al., "In Vivo Biocompatibility and Biostability of Modified Polyurethanes," Journal of Biomedical Materials Research, 36 (1997): 246–257.
KA White et al., "Surface Modification of Segmented Polyurethaneureas via Oligomeric End Groups Incorporated During Synthesis," Surface Modification of Polymeric Biomaterials, eds. BD Ratner and DG Castner (New York: Plenum Press, 1996).
D. Zhang et al., "Surface Studies of Polymer Blends by Sum Frequency Vibrational Spectroscopy, Atomic Force Microscopy, and Contact Angle Goniometry," Journal of Physical Chemistry, 102 (1998): 6225.
Meijs et al., Polysiloxane-containing polyurethane elastomeric compositions, WO98/13405 PCT Pat. Application, 1997.
Szycher et al., Hydrolytically and proteolytically stable polycarbonate polyurethane silicone copolymers, US Pat. 5,863,627, 1999.
Robert S. Ward is president of The Polymer Technology Group Inc. (Berkeley, CA), which provides biomaterials design/manufacturing and device/component development and manufacturing services to the medical industry. Work by the author related to the enhanced biostability of silicone-modified polyurethanes was gratefully supported by NIH-NIGMS SBIR Grant 1R44GM44539, Thermoplastic Siloxane-Urethane Copolymer Development, and NIH-NHLBI Grant 2R44HL5582402A1, Polymer Modification Via Surface-Modifying End Groups.
Return to the MDDI April table of contents | Return to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
You May Also Like

