How Scotch Tape Is Driving Diagnostics Breakthroughs
October 1, 2014
Something pretty similar to Scotch Tape could enable some major innovation in the IVD space--if a young St. Paul, MN-based company called Ativa Medical has its way.
Scotch Tape is something people in the Twin Cities know a lot about: The iconic product is made by 3M Co, based on the east side of the metro in Maplewood. And it is sheets of 3M adhesive polymer that provided a savvy solution for producing Ativa's business card-sized test cards for blood samples.
Researchers at the six-year-old company figured out how to laser-etch micro channels on tape, allowing for a single drop of blood to flow through the channels on a test card as the company's clock radio-sized MicroLAB device performs a variety of tests that include not only electrochemistry but also flow cytometry, imaging, and colorimetric analysis. The result is a complete blood count and a host of other tests that could be conducted within 5 minutes at a doctor's office, or any other place a blood test is being done, versus being shipped off to a central laboratory.
The microchannels on the test card are etched on multiple layers of tape that are then stuck together to create the card. Versus lab on a chip devices that require a silicon chip engineered for each test, Ativa officials think a tape roller at a manufacturer could easily spit out thousands of test cards a day--with each card selling for about $1.
|
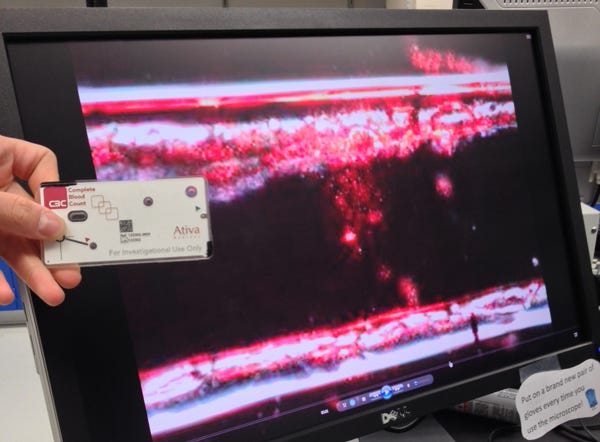
A computer monitor at Ativa Medical's St. Paul, MN lab shows how a single red blood cell (bright dot in center) flowing through a micro channel on the company's test card. Bob Janisch, Ativa's senior scientist, is holding a test card to the left. The micro channel is etched on multiple layers of 3M Co. adhesive polymer, similar to Scotch Tape, which are then stuck together. (Photo by Qmed senior editor Chris Newmarker) |
Better yet, the process is easily customizable. "We are able to create a prototype card for various assays within a day," says Bob Janisch, Ativa's senior scientist.
The channels on the single card replace all of the tubes one would find in a standard copying machine-sized machine where complete blood count tests are being conducted. Standard CBC machines require constant maintenance--and the need to avoid leaking tubes, according to Stephen Floe, director of corporate strategy at Ativa
"We've miniaturized and replaced the fluidics with a low cost disposable cartridge," Floe says.
"We took something this big," Floe said Tuesday morning, gesturing to an Abbott Cell-Dyn 3200 taking up almost a table in Ativa's University Enterprise Labs office in St. Paul, "and turned it into something this big," pointing to the MicroLAB. Shrinking so many testing devices into the MicroLab, as well as designing a laser that could quickly calibrate to follow blood cells flowing through the card, were other design challenges that Ativa researchers overcame.
Floe and Janisch acknowledge that smaller and even smartphone-based devices might eventually replace the original MicroLab for some tests, but the test cards and the proprietary method for producing them are an innovation that will last because of the way they can parse out minute quantities of blood flow for sampling. They showed off a video of single blood cells moving one at a time through a channel on the card.
Because the MicroLAB and its test cards are basically miniaturizations of tests already out there, the plan is for Ativa to seek a 510(k) with the FDA as a Class II medical device. The company, which has more than 20 employees and dozens of patents, over the summer hired a new president and CEO-- James M. McNally, PhD. McNally, who most recently served as vice president of Quest Diagnostics' Products Division, will lead Ativa through regulatory approval and manufacturing ramp-up.
Ativa is in the process of raising $7 million in a debt round, according to an U.S. Securities and Exchange Commission Form D from last year. The company raised $3.4 million in equity in 2012.
Refresh your medical device industry knowledge at MD&M Chicago, October 15-16, 2014, and MD&M Minneapolis, October 29-30, 2014. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like