How a Thermoplastic Beat Out Metal in a Laparoscopic Retractor
November 20, 2013
When medical device startup NovaTract Surgical Inc. (Madison, CT) set out to manufacture a disposable laparoscopic retraction system used to visualize and manipulate organs in various surgical applications, it could have formed some of the components out of metal. Instead, it opted for Ixef PARA, a 50% glass-filled grade of polyarylamide thermoplastic offered by Solvay Specialty Polymers (Alpharetta, GA).
|
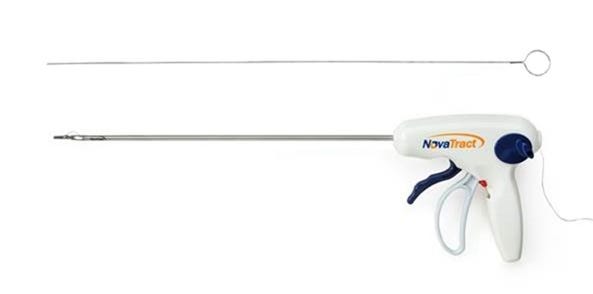
Solvay Specialty Polymers' Ixef PARA polyarylamide material replaces the use of metal in the trigger mechanism of NovaTract Surgical's disposable laparoscopic retractor. |
"Polyarylamide is partially similar to aromatic nylons, but it's a distinct type of material," explains Gilbert Galon, sales development manager, healthcare at Solvay Specialty Polymers. "What's unique about this material is that even with high glass loadings, it has a smooth, resin-rich surface. In addition, it is an extremely high-flow resin, so it can readily fill walls as thin as 0.5 mm."
In NovaTract Surgical's Dynamic Retractor, Ixef PARA is used to injection mold a 5-in.-long trigger mechanism. At ambient temperature, the material's tensile and flexural strength is comparable to that of many cast metals and alloys. Thus, it enables the trigger to withstand a high spring load, maximizing the stroke length of the retractor when it fires the device's nitinol and stainless-steel anchor. The advantage of using this material is its combination of strength and aesthetics, according to Galon. Moreover, the material is suitable for fabricating complex parts.
Cheaper and lighter than metal and ISO 10993:1-certified for biocompatibility, the material is suitable for replacing metal in disposable medical device applications, Galon says. "Used across the board in different industries to manufacture such components as office chair frames, electric shaver housings, and automotive parts, the polyarylamide material offers very low creep, good dimensional stability, a low rate of water absorption, and good chemical resistance." All in all, a sensible choice for mission-critical medical device components.
Bob Michaels is senior technical editor at UBM Canon.
About the Author(s)
You May Also Like