Developing Polyisoprene Materials that Meet USP Guidelines
July 14, 2014
By Saman Nanayakkara, Shu Peng, and Gino G. Banco
A new grade of polyisoprene compounds complies with USP <381> guidelines for both Type I and Type II elastomeric closures.
Natural rubber has been utilized in a variety of general industrial and commercial applications since the mid-1800s. In select applications, it has also found favor in the medical industry. Derived from the latex of the Pará tree, it is composed of long chains of isoprene monomers. However, the recognition that certain proteins in the raw material can elicit allergic reactions has posed a significant hurdle for medical device developers interested in exploiting the material's mechanical and physical properties in medical device applications, compelling them to search for alternatives.
This quest has led to the creation of synthetic rubber materials, including polyisoprene. However, while polyisoprene compounds are viable alternatives to natural rubber, traditional grades of this synthetic material do not meet all the requirements of U.S. Pharmacopeia's USP <381>, "Elastomeric Closures for Injections.1 This dilemma has created the need for a new polyisoprene compound that can meet the 2009 USP <381> Type I requirements. Such a material would lessen the regulatory burden facing medical device developers while providing superior resealability and other functional characteristics not typically achieved using other elastomeric materials
Why Polyisoprene?
In the mid-1950s, scientists discovered new types of catalyst systems that allowed for the development of synthetic polyisoprene rubber. Commonly referred to as polyisoprene, synthetic polyisoprene rubber is a cleaner, more consistent analog to its natural cousin. It does not contain proteins, eliminating the allergen issue that has limited the use of natural rubber in the medical device industry. Additionally, polyisoprene has a similar set of desirable mechanical, physical, and chemical characteristics as its natural counterpart, providing advantages for medical device applications.
Excellent material properties such as tear resistance, rebound resilience, elastic modulus, compression set, and stress relaxation can be achieved by compounding polyisoprene with carefully selected reinforcing fillers, plasticizers, cure systems, and other specialty ingredients. If formulated properly, polyisoprene compounds can meet USP Class VI and ISO 10993 biocompatibility guidelines.
Additionally, the material has the unique ability to reseal itself after being punctured, making it suitable for septa, or closures for injection fluid-transfer applications. When a needle pierces an elastomeric septum such as a vial cap closure or an IV bag port, it passes through the seal that protects the inner sterile environment from the outer environment. A properly designed elastomeric septum must allow easy needle penetration while maintaining the integrity of the seal at all times. It must also resist fragmenting, coring, or crumbling to prevent contamination and occlusions while exhibiting the ability to reestablish the seal when the needle is removed.
USP <381>-Compliant Polyisoprene
Despite polyisoprene's many beneficial properties, it is challenging to use traditional polyisoprene materials to manufacture medical injection closures because of stringent guidelines implemented by various standards organizations.2-4 For example, in "Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics," FDA states that the requirements presented in "USP Elastomeric Closures for Injection" are typically a baseline for demonstrating the safety of such components.5 Hence, any injection closure application submitted to FDA after May 2009 is expected to comply with the updated USP <381> guidelines.
The guidelines classify elastomeric closures into Type I closures for aqueous preparations and Type II closures for typically nonaqueous preparations. Type I closure requirements are more stringent than Type II requirements, especially for ultravoilet radiation absorbance and reducing substances. While certain traditional polyisoprene compounds meet Type II requirements, they have had difficulty meeting the updated physiochemical requirements for Type I closures.
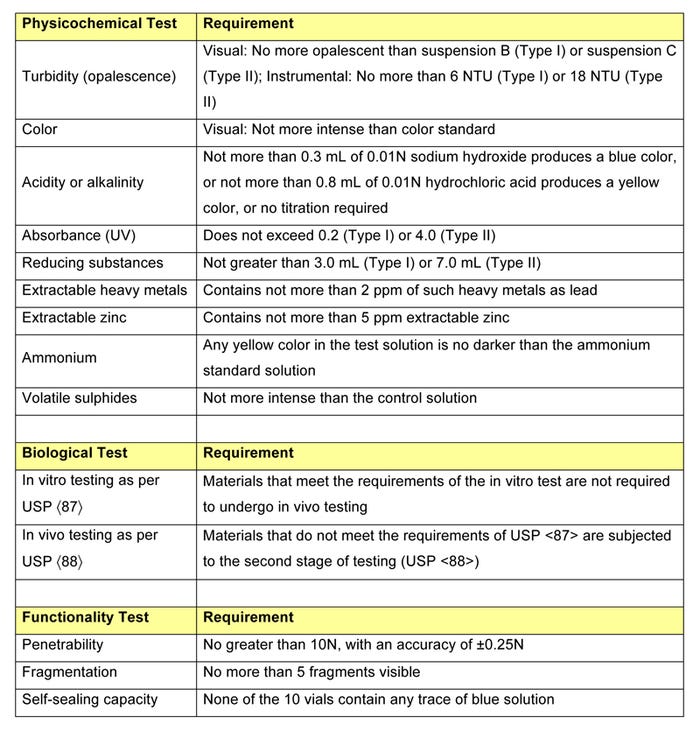
After undergoing extensive biological, physicochemical, and functional testing, a new polyisoprene compound, designated RJ651-30, has been shown to comply with USP <381> guidelines for both Type I and Type II closures. The requirements for each test are presented in Table 1.
|
Table 1: USP <381> testing requirements. |
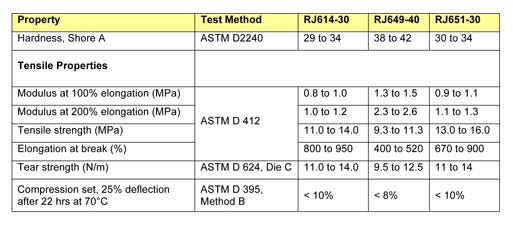
Table 2 compares the physical and mechanical properties of RJ651-30 with those of two traditional polyisoprene compounds that have historically been used in injection applications: RJ614-30 and RJ649-40. While these traditional compounds are USP <381> Type II compliant, they can no longer be used to manufacture closures for aqueous preparations because they cannot meet Type I physiochemical requirements. Since compound RJ651-30 is Type I compliant, it is by definition Type II compliant as well, rendering it suitable for use in both aqueous and nonaqueous preparations.
|
Table 2: Physical and mechanical properties of RJ614-30, RJ649-40, and RJ651-30 polyisoprene compounds. |
Ranging from 30 to 40 Shore A durometer, the hardness of the three compounds is typical for such materials. RJ614-30 and RJ651-30 both exhibit excellent tensile strength, high elongation, and very good tear strength. Compound RJ649-40, which exhibits the best compression set resistance, has slightly lower tensile properties and tear strength than the other two materials. All three compounds demonstrate comparable mechanical and physical properties and perform especially well in compression set resistance tests at 70°C.
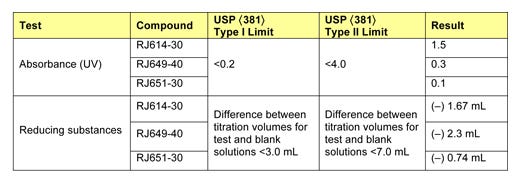
Table 3 presents selected results of physiochemical testing. In particular, the absorbance testing highlights the difference between the traditional compounds and the newly developed material. While the traditional compounds satisfy Type II guidelines for absorbance, they fail to meet Type I absorbance guidelines. In contrast, the newly developed compound meets Type I absorbance guidelines, with UV absorbance of 0.1 (50% of the limit).
|
Table 3: Physiochemical properties of RJ614-30, RJ649-40, and RJ651-30 polyisoprene compounds. |
Table 4 shows the results of functionality testing for RJ651-30, indicating that the material performs exceptionally well according to the functionality guidelines described in USP <381>. The penetrability, fragmentation, and self-sealing capacity tests were conducted using 21-gauge needles, as mandated in the guidelines.
|
Table 4: Functionality tests for RJ651-30 using a 21-gauge needle, per USP <381> guidelines. |
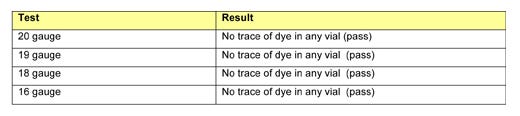
Since RJ651-30's self-sealing capacity performance exceeded the level required in USP <381>, this test test was extended to further examine the material's limits. First, the material was tested using needles measuring up to 16 gauge, the cross-sectional area of which is four times larger than that of a 21-gauge needle. The results of the self-sealing capacity tests performed using larger needles are presented in Table 5.
|
Table 5: Self-sealing capacity tests for RJ651-30 using 16- to 20-gauge needles. |
Next, the self-sealing capacity test was performed by piercing the vials 10 times using 20-gauge needles and then placing them under vacuum. Subsequently, the vials were stressed further by piercing them once, placing them immediately under vacuum, piercing them again, and placing them immediately under vacuum again. By repeating these steps with each vial until a failure was observed, more vial-to-vial resolution was achieved in the data, enabling the researchers to understand how many times the septa could be punctured and resealed before failure occurs. All but one of the 10 vials tested in this manner survived 20 cycles, while the failed vial succumbed at five cycles.
Conclusion
Polyisoprene compounds are well suited for use in medical septa because of their superior resealability and desirable mechanical, physical, and chemical characteristics. However, stringent USP guidelines have made it difficult to use traditional polyisoprene materials in elastomeric closures used for aqueous preparations. While traditional compounds can be formulated to meet Type II guidelines for nonaqueous preparations, they do not pass the absorbance requirements during physiochemical testing.
In contrast to traditional polyisoprene materials, a new polyisoprene compound provides proper mechanical and physical properties while complying with USP <381> Type I guidelines. The new material exhibits such good properties as low needle penetration resistance, unlikelihood of fragmenting or coring, and high resealability, making it suitable for injection septa applications. Because of its ability to comply with USP <381>, its excellent mechanical and physical properties, and its ability to incorporate properties from other polyisoprene compounds such as self-lubrication and antimicrobial agents, this compound shows that polyisoprene can still be used in injection septa.
References
USP <381>, "Elastomeric Closures for Injections" (Rockville, MD: U.S. Pharmacopeia, 2009).
EP 3.2.9, "Rubber Closures for Containers for Aqueous Parenteral Preparations, for Powders, and for Freeze-Dried Powders," European Pharmacopeia 5.0 (Strasbourg, France: European Pharmacopeia, 2005).
JP 7.03, "Test for Rubber Closure for Aqueous Infusions," Japanese Pharmacopeia, XV General Tests (Tokyo: Pharmaceuticals and Medical Devices Agency, 2006).
ISO 8871, "Elastomeric Parts for Parenterals and for Devices for Pharmaceutical Use" (Geneva: International Organization for Standardization, 2005).
"Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics" (Silver Spring, MD: U.S. Food and Drug Administration, 1999).
Saman Nanayakkara is a laboratory manager and senior chemist at Parker Hannifin Corp.,'s Medical Systems Div. (Anaheim, CA). He joined the company in 2009. A polymer engineer, he has more than 25 years of experience in elastomer compounding, characterization, testing, and processing. Contact him at [email protected].
Shu Peng is engineering manager at Parker Hannifin Corp.'s Medical Systems Div. He joined the company as a senior development engineer in 1992. He specializes in polymer engineering, rubber elasticity, and finite element analysis. Contact him at [email protected].
Gino G. Banco serves as principal R&D engineer in Parker Hannifin Corp.'s Engineered Materials Group. He joined the company in 2010. His focus is on the development of products and technologies for the life sciences market. In this capacity, Banco works with the Engineered Materials Group's 14 divisions, including the Medical Systems Div., leading global teams in new materials development, manufacturing, and medical device initiatives. Contact him at [email protected].
About the Author(s)
You May Also Like