Developing Fully Open-Cell Silicones for Implantable Devices
October 15, 2014
Biocompatible, chemically inert, and sterilizable, open-cell silicones made using a new manufacturing process are suitable for both short- and long-term implantable applications.
In their quest for new medical device materials, designers, developers, and manufacturers have a choice between open- and closed-cell structures. However, while open-cell materials have a range of advantages over their closed-cell counterparts, one of the most common open-cell materials--polyurethane--is primarily used in nonimplantable settings for such products as cleaning devices, wipes, or absorbent pads.
Silicones, on the other hand, are biocompatible, but they are generally available only as closed-cell structures, rendering them unsuitable for implantable applications. And while so-called 'open-cell silicone' is available commercially from various manufacturers, these products are not fully open-cell structures. They contain a large number of closed cells together with nonporous skins.
Solaris Technology Group (San Diego) is endeavoring to rewrite this scenario. It has developed a manufacturing process that can produce fully open-cell silicones that are suitable for a range of implantable medical device applications.
|
Polyurethane open-cell material is generally not suitable for implantable medical device applications. |
"Open-cell polyurethane foams are primarily used outside the body or for short periods of time within the body to absorb or wick bodily liquids," remarks Nick J. Manesis, president of Solaris. "They are generally not used in implants that will be exposed directly to tissue. Moreover, the material is known to degrade completely in the body within one to two years--not a positive attribute." In fact, aesthetic implants covered with open-cell polyurethane were removed from the U.S. market in the 1990s because of concerns that they could degrade into potentially carcinogenic by-products, increase the risk of infection, and leave polyurethane foam remnants during explantation.
In contrast, open-cell silicone can be made into stable, biocompatible structures, according to Manesis. Hence, they are suitable for both short- and long-term implantable devices or for implant fixation and positioning applications. In addition, they can play an important role in tissue integration, tissue scaffolding, and cell seeding. Chemically inert and sterilizable using gamma, E-beam, EtO, dry heat, and autoclaving, these materials offer improved gas and liquid exchange while decreasing fouling or the absorption of components at the material-tissue interface.
|
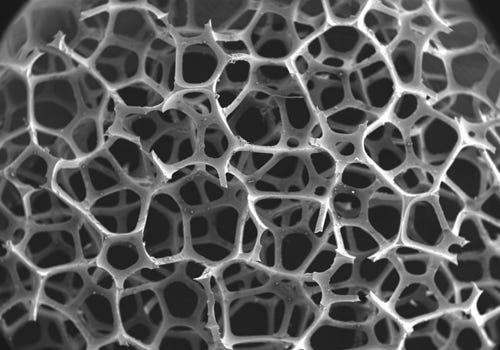
Suitable for implantable applications, Solaris's open-cell silicone features multiple interconnections between the cells, allowing it to be formed into a 3-D network. |
"Open-cell structures are materials that have been formed or fabricated into a 3-D network," Manesis explains. "This network is extremely porous and features multiple interconnections between the cells. The communication between the cells gives rise to a low-density structure with struts or supports composed of the desired material."
For example, polyurethane foams are organic polymer-based materials that contain cross-linked urethane linkages, Manesis says. Depending on the application and the properties desired, these foams can be made from polyester and polyether chemistries. Various physical properties can be engineered into the materials, including the number of pores per linear inch, foam density, cell and interconnection diameter, the number of interconnections per cell, and such mechanical properties as hardness, tensile strength, and compression.
The open-cell nature of commercial polyurethane foams is produced using blowing agents present during the manufacturing process. Often formed in situ, these agents include carbon dioxide or a low-boiling liquid. However, after polymerization, not all of the material's cells are open, Manesis comments. "To break 'windows' between the cells and obtain a true open-cell structure, a secondary process must be performed. Known as cell opening, this process involves either a mechanical treatment using explosive hydrogen gas or a chemical treatment using sodium hydroxide.
|
Commercially available open-cell silicone material does not contain 100% open cells. |
Like open-cell polyurethane foams, existing commercial grades of open-cell silicones do not contain 100% open cells. Moreover, they have poor mechanical properties because they comprise only a fraction of the original silicone from which they were produced. For example, current open-cell silicone foams break when elongated by 30%, while the base material can be elongated by more than 1000% before breaking.
In contrast to currently existing processes, the Solaris open-cell interconnected silicone (OCI) process can produce 100% open-celled, high porosity silicones, Manesis notes. At the same time, the cells are not produced using blowing agents. Furthermore, these structures retain the mechanical properties of the original material. Depending on the type of silicone used, elongation of more than 1000% can commonly be achieved before the material breaks.
Currently, open-cell materials have not gained ground in the medical device space, Manesis laments. The reasons for this include the lack of available technologies and their commercial presence. Moreover, the types of chemistries used to manufacture polyurethane foams limit development and product opportunities. However, understanding the advantages of using open-cell silicones in implantable applications, Solaris is making headway in the commercialization of these materials. The company, according to Manesis, has developed a range of open-cell silicone foam prototypes with various porosities, interconnectivities, pore sizes, and interconnection diameters, advancing its products to the industrialization stage.
Bob Michaels is senior technical editor at UBM Canon.
About the Author(s)
You May Also Like