Balloons Feature Latex Properties without the Risk
Wireless data collected directly from patients during normal physical activities could enable the development of better knee implants
April 7, 2008
Originally Published MPMN April 2008
BREAKTHROUGHS
Balloons Feature Latex Properties without the Risk
|
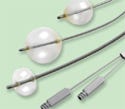
Medical balloons made from latex extracted from a desert plant are safe for people with Type I latex allergy. |
Conventional latex is used for a plethora of medical products ranging from disposable gloves to catheters. But, with latex allergy statistics continuing to rise, a host of hospitals are initiating a push to purge conventional latex from the healthcare setting. Tailoring its offerings to meet this emerging demand, TechDevice Corp. has introduced minimally invasive medical balloons that boast the desirable characteristics of latex without the health risk.
The balloon material comes courtesy of Yulex Corp., which stakes its claim as the first company to commercially extract latex from the desert plant guayule. The natural rubber latex extracted from guayule does not contain the proteins found in Hevea (traditional) latex, which are believed to be the allergen trigger. As a result, the firm has found that the material is safe for people suffering from a Type I latex allergy. Following a four-year collaboration focused on developing balloons from the material, TechDevice signed an agreement for the exclusive manufacture of Yulex latex–based medical balloons for catheter-related products.
Common balloon materials include polyurethane, polyethylene, silicone, and nylon. But, health risks aside, latex is a favorable material for use in these devices because of its distinct properties, according to Michael Brown, TechDevice director of operations. “Latex just happens to have certain properties that are hard to duplicate,” he says. “It is very elastic and [when used for balloons] you can get very thin wall thicknesses as well as a low profile at the end of a catheter.”
The guayule latex balloons feature these characteristics and also have tensile strength and durability that is better than those made from high-end synthetic products, according to the company. “The guayule latex formulation used for medical applications has been carefully engineered to replicate the properties of Hevea latex,” says Leigh Hayward, TechDevice director of technical operations.
As the only company offering Yulex balloons, TechDevice is now setting its sights on spreading the word about its balloons, highlighting the benefits they provide to both patients and OEMs. “There are all of the existing latex balloons that have the Hevea latex, and obviously the allergy associated with it. There is an opportunity to replace everything on the market with Yulex balloons,” speculates Brown. “There’s also a market for new devices that probably are using different materials, such as a silicone, because Yulex latex wasn’t previously available.”
TechDevice Corp., Watertown, MA
www.techdevice.com
Copyright ©2008 Medical Product Manufacturing News
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

