A Cutting-Edge Biomaterials Technology with 19th Century Roots
March 27, 2014
Imagine the case of a man involved in a motorcycle accident who ends up with a gash that severs the nerves in his arm. Using a biomaterial scaffold loaded with growth factors, the nerve could be coaxed into fusing back together.
Or imagine the use of vascular grafts that can trigger blood vessel growth that could be used in place of stents.
Orchestrating nerve regeneration, blood vessel repair, and wound healing are among the many potential applications of Dallas-based TissueGen's Elute biodegradable fiber products, which encompass biodegradable polymers such as poly(L-lactic acid), poly(p-dioxanone), poly(L-lactic-co-glycolic acid), and poly(D,L-lactic acid).
And the fibers are all possible thanks to a novel and patented modification of a production process that has been around since Abraham Lincoln was alive.
In an interview with MPMN, the company's founder and chief scientific officer Kevin Nelson and CEO Christopher Knowles, CEO of TissueGen explained how the technology works:
|
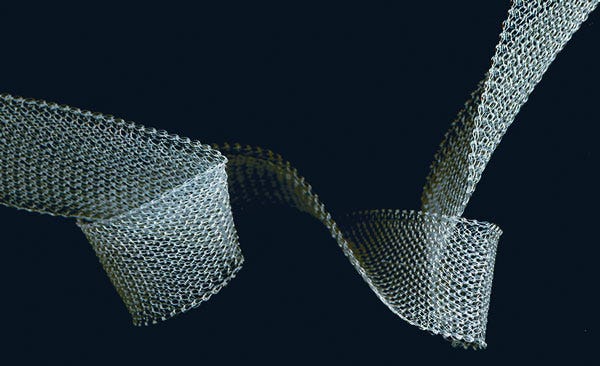
TissueGen's absorbable biomaterial technology can be used for an array of implantable drug delivery applications. |
MPMN: What sets your technology apart from other biomaterials that are on the market?
Nelson: What we bring to the board is the ability to take standby materials and load drug into them and, within that standardized format, tailor the release kinetics. We can achieve what we do by simply making alterations in either the porosity of the fiber or in the degree of interaction that the excipient has with the surrounding polymer matrix. With those two basic variables, we can change the release kinetics of the drug significantly. Just as an example, if we make a relatively porous fiber, we can have drug release in a matter of a week. If we make that exact same fiber and drug, but we reduce the porosity, that drug delivery is now out many weeks--months in some cases.
MPMN: How does the company avoid damaging the drug or growth factors when producing the fiber?
Nelson: We use wet extrusion, a technology that has been around since the 1850s. In fact, it was the first way that people thought of as making a synthetic fiber. The theory of it hasn't changed obviously since then, and wet extrusion has a lot of commercial applications in non-medical applications. Kevlar, Spandex, Acrylic, and Rayon are polymers that are made with the technique.
Within the medical field, however, its use is quite novel.
Knowles: Wet extrusion is a time-proven technology but what is novel and unique about our use of it is the combination of the process with biomaterials.
MPMN: What are the advantages of using fibers made from wet extrusion in the medical field and could you briefly describe how it compares with process used to produce most polymer fibers.
Nelson: For most polymers, what you do is have a big screw and the screw crushes and heats the polymer and pushes the polymer, which melts as it travels down the screw because the screw has got heaters on it. By the time it emerges, it has melted and at an extremely high temperature and generally extremely high shear rates. That is very low cost. Once you by the screw and plug in the heater, that is pretty much it for your costs. Therefore the way that probably 90% of the world's polymers are extruded by that process.
Wet extrusion on the other hand differs dramatically but in many respects, they are very similar. The goal is coming out of the spinneret as a liquid. In the melt extrusion process, you achieve the liquid state by heating it. In wet extrusion, you achieve the liquid state by redissolving the polymer in a solvent. And polymer-solvent combination is pushed through your spinneret. Then that is pushed into a coagulating bath and the essence of the coagulating bath is that it is filled with a liquid that has the requirement of being infinitely miscible with the solvent that you dissolved the polymer in, but at the same time a very poor solvent for the actual polymer.
What happens is that as your liquid stream runs into the coagulating bath, because the two solvents are infinitely miscible, the good solvent will dissolve out of the bath and that bad solvent will start to diffuse in. When the polymer molecules see the bad solvent, they begin to precipitate on each other and eventually will form a fiber. The good solvent diffuses out, and the bad solvent precipitates it and the fiber is formed. By the time you get out of the coagulating bath, you now have a solid fiber. Similar to hot extrusion, that solid fiber is going to go down an extrusion line where it is pulled on to align the polymer to make the fiber stronger.
To compare and contrast, on the melt extrusion process, you put it through an oven that remelts it and in that molten state, you can now pull the fiber and the polymer molecules become aligned. Then it cools off. And you remelt and draw it repeatedly, five or six times, and each time the molecules get more and more aligned and the fiber gets stronger.
The wet extrusion line looks identical. If you took a picture of the two lines, you couldn't tell the difference. There will be drawing stations and intervening ovens. The difference is that in wet extrusion you don't need to add a lot of heat. There is still enough residual solvent around that the alignment can be largely made with very low heat. While it is true we have ovens and drying machines, our ovens are heated to 40°C. That is the temperature of a high fever when you are sick. It is all within tolerable body temperature throughout the entire process. We can achieve through wet extrusion similar kinds of mechanical strength that you can get with the melt drawn fiber but we do so all at very low temperature. The growth factor, the proteins, and the drugs that we put in there can all deal just fine with a 40°C incubation for a few seconds.
Knowles: What that is relevant is that through a hot extrusion process, you destroy most of the biologics and the drugs.
Conversely, what is interesting about common wet extrusion is that it requires the use of harsh chemicals, which also destroy most drugs and biologics. TissueGen's technology, however, allows the drugs and biologics enables the delivery of drugs and biologics through a fiber.
Nelson: The IP that we have, our patents and internal trade secrets, is all around preserving the biological activity of the molecule during this wet extrusion process. In wet extrusion, it is possible because you can protect that drug, protein, or whatever it is from solvents relatively easily. You cannot protect most drugs from temperatures of 100°C. There are a small handful of drugs, such as some types of antibiotics, can in fact survive that 100°C hot melt extrusion process. There have been papers going back to the 1960s about putting tetracycline in a suture in a melt extrusion process and that works just fine.
|
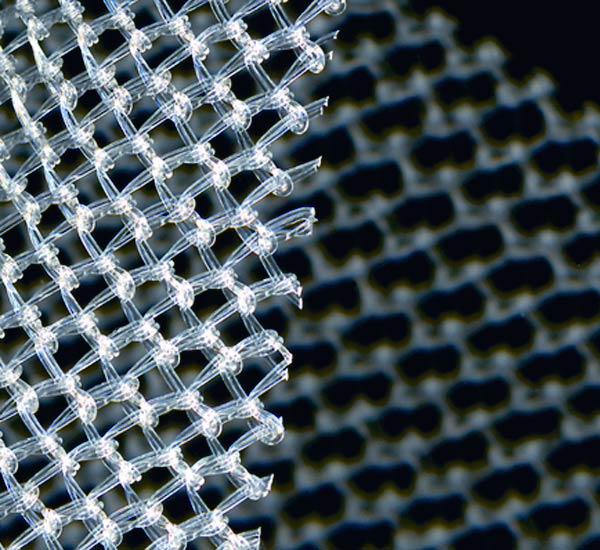
TissueGen's biomaterials can be loaded with protein-based biologics such as growth factors and enzymes, as well as conventional pharmaceuticals. |
MPMN: Can you summarize how your technology be used to orchestrate nerve regrowth?
Nelson: We have the technology to create a fiber featuring a three-dimensional concentration gradient. It is quite easy to make a one-dimensional concentration gradient, meaning we can extrude the fiber so that along the length of the fiber, the concentration goes high low, high low, whatever. Then you can cut it and say: 'on this six inch piece of fiber, it is lower at this end and higher at this end.' You can create a concentration gradient of a drug one dimensionally.
This functionality is useful for nerve regrowth. If you think back to your seventh grade science class, if you have a little bowl with bacteria or some other one-cell animals in it, and I put a drop of sugar on one side of the bowl, it doesn't take long for all of the bacteria to swim over to corner with the sugar.
A similar principle is at work in our nerves. Injured nerves can detect growth factor similar to how bacteria detect sugar. The peripheral nervous system is inherently regenerative, which is why when you get a paper cut, you don't lose sensory information for the rest of your life. The nerves grow right back.
Find out more about the medical device industry--including biomaterials, technology, supplier networks, and cutting-edge technologies like 3-D printing much more--at MD&M East held June 10-12, 2014. |
If you have someone who has had a motorcycle, and they have broken the main branch that goes down the right arm, most of the time when that kind of injury takes place, the nerve will retract on both sides. When the doctor in the OR tries to go in to suture the motorcycle's arm back together again, sometimes he is lucky enough to actually bring the two nerve stump ends back and suture them together. Frequently, he can't. If he pulls to hard to stretch them to bring them back together, they don't function as well.
Current medical treatment is to go someplace else on that person's body, harvest a nerve of the right diameter from somewhere else in the body. So that is current medical best treatment, which is not desirable if you are the patient. You don't want to wake up minus feeling in a body part that wasn't injured anyway.
Back to the example of a paper cut. If my two nerves are close together, the downstream side emits a number of proteins--growth factors that the upstream neurons can 'smell,' so to speak. They can sense the presence of that growth factor and say 'oh, that's my signal and I can follow the concentration gradient and reconnect.'
You can imagine that as that gap gets further and further apart, the upstream nerves become less capable of detecting that growth factor that the downstream side is emitting. Once you reach a point where there is no detected signal, and the upstream neurons are inherently regenerative, they want to grow someplace but they randomly grow in several directions, which leads to sensory neurons going to places they shouldn't, which can lead to a lot of pain for the patient and it leads to dysfunction in terms of the muscles or organs that were supposed to be innervated by that nerve or not.
What we bring to the board is an ability for our fibers to emit that exact same growth factor, which can be delivered right to the two stumps of the nerve in the peripheral nervous system. The upstream side will say: 'oh, I detect growth factor.' But that's not because it's being emitted by the cells that are a long ways away but because it is being released by the fibers they are touching. The fibers give the signal to the nerve and say, 'hey guys, you want to grow. So they give them the right growth factor and also provide the physical scaffold.' That leads the nerves mechanically to this end to that end giving you the stimulus of the growth factor that is telling you it is going in the right direction.
In the case of nerve regeneration, that is the basis for how and why this works. On a short gap, a non concentration gradient is probably fine. But as that gap gets longer and longer, you probably will need to induce a three-dimensional gradient to channel the nerve cells in the direction they need to go.
MPMN: It seems your technology can be used for a wide array of applications, including custom drug delivery, cardiology, dermal wound healing, nerve regeneration, sutures, vascular stents and grafts, orthopedics, and general surgery. Your strategy is to use one product line and go after many applications?
Knowles: Yes. That one material has many applications. Going back to the beginning of last year, there was a decision to commercialize that core material. It fits in with our position to offer the biodegradable drug loaded fiber or BDL fiber. We focused on commercialization and sales of that product. It is an interesting marketing strategy because it is one a one-product strategy targeting a myriad of potential applications in different fields of medicine. For instance, we are working on spinal cord injury repair. We are also working on braiding a small diameter vascular graft for applications. Vascular stents could be replaced perhaps by larger diameter vascular grafts. The idea that you can create blood vessels and now weave 3-D architecture with relevant growth factors that will allow these synthetic structures to endothelialize while at the same time, the fiber itself eventually goes away and encourages your body to grow new vascular tissue.
Brian Buntz is the editor-in-chief of MPMN. Follow him on Twitter at @brian_buntz and Google+.
About the Author(s)
You May Also Like