2007 MEDTECH SNAPSHOT
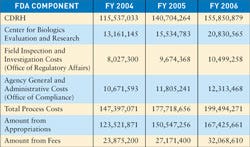
Last year, MD&DI used preliminary reports from MDUFMA to generate findings for its 2006 December issue. The MDUFMA reports released for FY 2006 confirmed those preliminary numbers and are reprinted in this section. CDRH did release its FY 2006 annual report, and therefore, data from that report regarding the Third-Party Review Program have been updated.
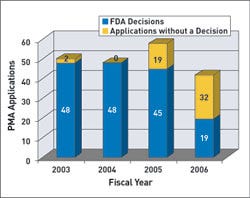
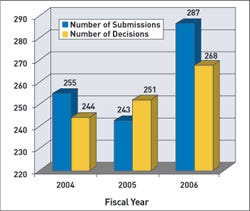
For premarket approvals (PMAs), cycle goals apply to 75% of submissions received in FY 2005, 80% of those received in 2006, and 90% of submissions in 2007. FDA's goals state that it will make decisions on 80% of PMAs received in FY 2006 and 90% in FY 2007. FDA has said it will have a decision for 75% of submissions received in FY 2005 and FY 2006. For FY 2005, FDA plans to apply these goals to 70% of the submissions. In 2006, it will include 80% of those submissions, and in 2007 the goals will apply to 90%.
Click images and tables to enlarge:
Copyright ©2007 Medical Device & Diagnostic Industry