A Drowning Medtech Company Clings to a Life Preserver
Asensus Surgical was once poised to take on Intuitive Surgical. Now the company clings to a life preserver from a prospective buyer.
April 9, 2024

The company that once hoped to take on Intuitive Surgical and “revolutionize the robotic market” is currently clinging to a life preserver from Karl Storz.
As MD+DI reported last week, Karl Storz and Asensus Surgical are in negotiations for a potential acquisition. The companies signed a letter of intent that includes an exclusivity period of up to 10 weeks. The problem is that as of March 21, Asensus just barely had enough available cash to survive that long.
The Durham, NC-based company ended 2023 with about $21.1 million in cash equivalents and short-term investments, excluding restricted cash. In the third quarter of 2023, Asensus initiated a cash burn reduction plan to streamline expenses.
“Based on the recent financing and our current operating plan, we project that available cash will now sustain operations until early June,” CFO Shameze Rampertab said during the company’s earnings call March 21.
But German medical device maker Karl Storz also tossed Asensus a life preserver in the form of a loan. During the 10-week exclusivity period, Karl Storz will loan Asensus up to $10 million. Then, if a merger agreement is reached, Asensus can borrow up to $10 million more while it engages with stockholders for approval.
Navigating choppy waters
Back when Asensus was known as Transenterix, the company had high hopes for bringing its SurgiBot to the U.S. robotic surgery market in 2016. Designed to enable the surgeon to perform single-port laparoscopic procedures at the patient’s side with robotic assistance, the SurgiBot was considered one of the most anticipated devices that year. If cleared, the system would have been the first to compete directly with Intuitive Surgical.
But in April 2016, FDA rejected the company’s 510(k) clearance submission for the SurgiBot. The agency decided that SurgiBot was not substantially equivalent to a predicate device, and that a new application would be needed.
So, the company turned its attention to a different technology, the Senhance, which was previously called the Alf-x.
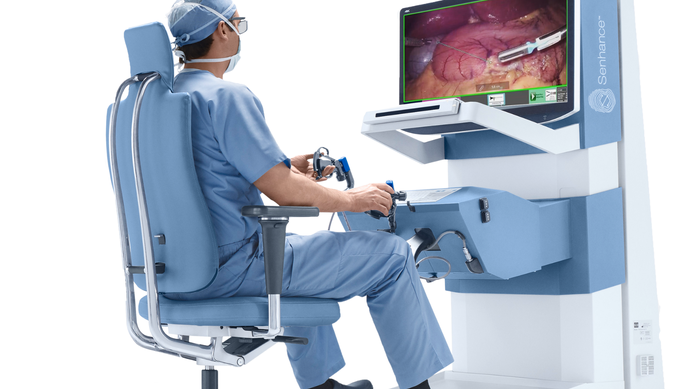
Surgeon using the Senhance System. Image courtesy of Asensus Surgical.
Then-CEO Todd Pope told me at the time that surgeon feedback touting the way the system “enhances their senses” inspired the company to rebrand Alf-x as Senhance. Haptic feedback was one of the biggest selling points of the system. The company also emphasized throughout the development process that the system would enhance the surgeon’s performance, not replace the surgeon. The Senhance also included a 3D camera and eye-tracking feature that allows the surgeon to move the camera with their eyes instead of having to rely on someone else to drive the camera or having to disengage from the operating arms to reposition the camera for a better view.
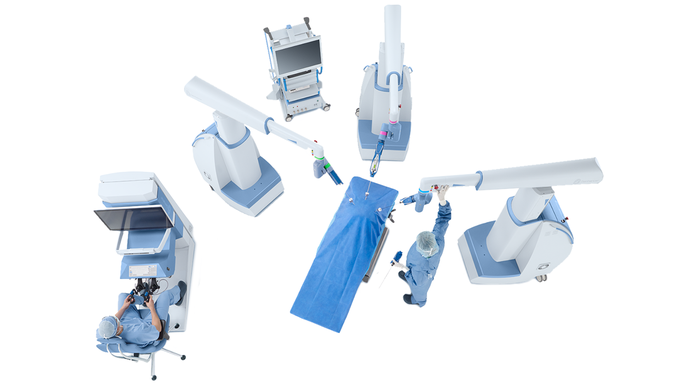
Bird's eye view of the Senhance System. Image courtesy of Asensus Surgical.
Despite these design features, the Senhance failed to consistently live up to the high expectations of physicians using Intuitive Surgical’s da Vinci technology.
Facing disappointing sales, Pope resigned, and CEO Anthony Fernando took the helm in November 2019. Then, in February 2021, the company changed its name to Asensus Surgical. The rational given for the rebranding was that the new name reflected the company’s broader vision of shaping the future of surgery by integrating computer vision and machine learning with surgical robotics.
Fernando joined MD+DI’s podcast, Let’s Talk Medtech, in April 2021 to talk about the change and why the firm saw itself as so much more than a surgical robotics company.
Luna development timeline adjusted
Asensus is currently working on Luna, its next-generation surgical system. To conserve capital, the company has adjusted the development timeline of the new system.
"This decision allows us to maintain physical stability while still advancing towards key milestones for Luna," Fernando said during the company's earnings call in March. "We now intend to freeze the systems design in Q3 2024 and conduct verification and validation testing in Q4 2024. Subsequently, we will initiate pilot manufacturing and prepare for regulatory submissions. The submission is now expected in the second half of 2025 with clearance anticipated in the first half of 2026, followed by a launch thereafter."
Fernando said the company plans to follow a traditional 510(k) pathway in the United States rather than the more rigorous de novo pathway. This, he said, should save both time and money.
In December, Asensus hosted a surgeon lab to conduct an in-vivo evaluation of Luna's hardware, software, and instruments in pigs. Fernando said the lab allowed nine participating surgeons to evaluate Luna's functionality in 13 different procedures across gynecology, urology, and general surgery. See video below (courtesy of Asensus Surgical) to hear about the design of Luna and surgeon feedback from the lab.
"The surgeon lab highlighted Luna's notable range of motion and instrument dexterity essential or precise surgical maneuvers," Fernando said. "Surgeons also appreciated the strength and reliability of the 5-millimeter true risk instrument line offering versatility with different instrument types. Additionally, they found the ergonomic design of the Luna surgeon console and easy patient access to be noteworthy features."
The CEO said insights gathered during the lab will aid in refining the system design.
About the Author(s)
You May Also Like




