In Vitro Diagnostics: Bringing Testing to the Point of Care
Medical Device & Diagnostic Industry MagazineMDDI Article IndexCover StoryAdvanced IVD instrumentation promises quicker turnaround for patient testing.
April 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Cover Story
Advanced IVD instrumentation promises quicker turnaround for patient testing.
|
|
The Careside Analyzer (Careside Inc.; Culver City, CA) and a CAD/CAM image of the device with the outer "skin" removed. |
During the past 25 years, diagnostic testing has slowly but steadily moved out of the central laboratory and into testing sites closer to patients. This testing modality, referred to as point-of-care (POC) testing, has enabled laboratory service providers to perform testing anywhere the patient is located, including outpatient clinics, nursing homes, alternate-care centers, the patient's home, and at the hospital bedside. In recent years, dramatic technological innovations in in vitro diagnostic (IVD) testing have enabled healthcare providers to deal more easily with a number of challenges, including analytical performance requirements, compliance issues, and rising costs.
This transition from laboratory to POC testing is bringing about a fundamental restructuring of the ways that the medical community delivers patient care. Traditionally, testing has been conducted using central laboratories in a hospital setting or at a commercial reference laboratory. In most cases, central labs require 4–24 hours to provide results for routine tests, while reference labs can require as much as 48 hours—barring such mistakes as lost specimens, specimen mislabeling, and laboratory accidents. Healthcare provider organizations have expressed frustration over the inherent costs and inefficiencies associated with such slow turnaround time for testing, and have sought to implement changes in medical practice designed, in part, to enable the organizations to remain viable and profitable while providing first-rate healthcare.
As a result, physicians have begun to adopt advanced technologies and care strategies that can reduce the time and cost needed to obtain test results. While continuing to provide accurate results, clinicians have begun to use POC testing to shorten patient visits and stays; increase patient satisfaction; and improve diagnostic, therapeutic, and monitoring practices. The growth of home-care and outpatient services is a direct result of these changes in the market and continuous pressure to treat patients outside of costly acute-care facilities.
To illustrate the kinds of technological advances that are revolutionizing point-of-care testing, this article focuses on the timely example of the Careside Analyzer, which FDA cleared in December 1999 for POC testing applications in the United States. For ongoing coverage of the IVD field, readers should visit the Web site of MD&DI's sister publication, IVD Technology. |
The U.S. market for sales of such POC testing devices was estimated at $2.4 billion in 1998. Over the next five years, the market for POC devices is expected to reach $4 billion in product sales.
CLINICAL CHALLENGES
Clinicians desire fast, easy-to-use, cost-effective, and accurate blood-testing devices that can be used at the point of patient care. To be successful in today's marketplace, such devices must address the needs of healthcare payers, provider organizations, caregivers, laboratorians, and patients. Following are some of the key parameters that are guiding the development of the current generation of POC test instrumentation.
Cost-Effective Results. The demand to curb rising healthcare costs, coupled with the government's unrelenting drive to reduce reimbursement rates for laboratory tests, has altered the delivery of testing services. For POC testing to be widely utilized, the cost per reportable result must be the lowest option available to the healthcare provider and payer. The device must also be available at a purchase price acceptable to the purchaser or via a lease option. The typical price of a POC testing device is less than $10,000, but can vary greatly according to its features and test menu.
Comprehensive Test Menu. To provide the majority of diagnostic information necessary for a physician to make a medical decision, the POC system must offer a broad menu of the most commonly ordered blood tests in the areas of chemistry, coagulation, immunochemistry, and hematology. A menu of 70 routine tests representing these four areas can cover more than 90% of the testing needs of alternate-care sites and 60% of the needs of hospitals.
Ability to Meet Regulatory Requirements. The Clinical Laboratory Improvement Amendments of 1988 (CLIA) set forth the regulations governing the performance of laboratory testing in the United States. CLIA defines three levels of testing complexity (waived, moderate, and high complexity), each requiring a different type of application and compliance. Approximately 75% of all tests performed in laboratories today—including the majority of POC tests—fall into the category of moderate complexity. To be commercially successful, it is essential that the POC device make CLIA compliance as easy as possible through automatic documentation, quality control checks, and user-interface prompts. At the same time, access to the test instrument must be controlled and documented so that only personnel properly trained to operate the device may do so.
Accuracy. The accuracy and precision of the POC instrument must be equivalent to those that can be achieved by large testing devices used by central laboratories. As a guideline, the total error represented by the POC test's bias and coefficient of variation should not exceed one-third of the CLIA analytical quality requirement for that test. For a cholesterol test, for example, the total error should ideally be no more than 3.3% at a medical decision limit of 200 mg/dl. For a glucose test, the acceptable total error is 3.3% at a medical decision limit of 120 mg/dl.
Quality Control. Collection of quality control data must be simple and low-cost. Ideally, data should be collected in the routine course of test performance by the same personnel that produce results in the patient-care setting. Data should be summarized in a form that makes review and identification of unacceptable results simple to recognize. And results should be saved in a form of documentation acceptable to the laboratory director and licensing agency or accrediting body.
Quality Assurance. Quality assurance should involve regular assessment of all aspects of the POC testing process, and appropriate response to problems that may be encountered. The assessment should include both instrument function and operator proficiency. The POC device should assist the user in documenting and controlling the preanalytical, analytical, and postanalytical testing phases, thereby aiding in overall quality assurance.
Ease of Use. The POC device must be simple to operate and easy to learn. In settings such as alternate-care sites, the device may normally be operated by nonlaboratory staff, often with no more preparation than a high school diploma. Turnover of such individuals can be rapid, so training time needs to be kept to a minimum—ideally, 30 minutes or less. Such staff also require a user interface that is intuitive and graphic. Similarly, the amount of operator decision-making should be minimal: the device should not require reagent preparation, specimen preparation, quantitative specimen transfer, or calibration. Autocalibration with reagent stability of 12 months or more is a common prerequisite. Among clinicians, whole anticoagulated blood is the specimen of choice. They also favor instruments that require only a small sample volume—less than 100 µl (3 drops of blood)—whether for single or multiple tests. Every precaution should be taken to protect the user from exposure to biohazardous material. Closed-tube sampling is a highly desirable feature.

Top Left: Exploratory sketches from the early design phase of the Careside Analyzer. Top Right: Early prototype of the analyzer utilized a separate keyboard later abandoned in favor of a touch screen, as shown in the final version (Bottom Right). Functions and features of the analyzer can be customized using the touch-screen user interface (middle). After the patient sample has been added, the cartridge is sealed and inserted into the analyzer (Left Bottom).
Information System Interface. As more testing is transferred from central laboratories to POC locations, the volume of information handled by POC devices will expand exponentially. The POC device must be able to capture, store, and transfer to an information system a variety of data including patient identification, diagnostic codes (ICD-9 codes), billing, test results, quality control, and quality assurance. This information should be transferred in real time via wired or wireless technology to laboratory information systems (LIS), hospital information systems (HIS), and physician practice systems. The connectivity of POC devices will be of paramount importance in the next two years.
Speed. When it comes to reporting accurate results to the caregiver, time is of the essence. Quick turnaround time in the reporting of a hematocrit level can mean the difference between a patient bleeding to death due to occult internal hemorrhaging and a life-saving transfusion. In emergency situations, the results of tests such as those for blood glucose, hemoglobin, hematocrit, arterial pH, and blood gases, are required in minutes. Other measurements, such as complete blood count, electrolytes, prothrombin times, and ammonia, are necessary for urgent-care situations and must be delivered within hours. Results from POC devices should be available in less than 15 minutes from time of specimen blood draw to final result.
Size. The instrument needs to fit the limited space available at POC testing sites. The instrument should be a stand-alone device capable of operating under a variety of conditions and power constraints. The reagent packs, such as disposable test cartridges, need to be small with long shelf lives. Room-temperature storage is highly desirable.
TECHNOLOGY RESPONDS
We are living in an information age where faster is better: faster computers, faster communication, and of course, faster diagnostics. Healthcare providers (and patients themselves) want to know as soon as possible the condition of the patient—whether healthy or sick, in or out of control, trending up or down. To date, central laboratories have been charged with the responsibility of providing such diagnostic information, and have chosen to do so by means of batch processing systems using large, complex, technology-specific testing devices. However, advances in microelectronics, microfluidics, and microfabrication are enabling diagnostics manufacturers to break this mindset and create a new generation of small, portable devices that provide instant results wherever the patient is located.
One example, which received FDA clearance in December 1999 for POC use, is the Careside Analyzer, an automated POC blood-testing instrument that makes use of testing technologies similar to those used in hospitals and commercial laboratories—only smaller. The analyzer measures approximately 13 x 12 x 11 in. and weighs about 20 lb. Its exterior is made of high-impact plastic. The top of the instrument consists primarily of a touch screen installed at a 60° ergonometric angle, on which the user inputs patient, physician, and billing information, the tests to be conducted, and any desired commentary. The graphical user interface is tailored to maximize ease of use. The testing and quality control process was carefully evaluated to provide simplicity of use and the most efficient use of information. The instrument is UL 2601–approved for use near patients.
This new device responds to the needs of clinicians by eliminating many of the steps involved in central laboratory blood testing, including the transport, accessioning, aliquotting, and preparation of specimens; the review of batch quality control records; and the communication of results. The device provides test results within 6 to 12 minutes, and can also perform blood tests on a stat, or immediate, basis. In a number of key areas, this analyzer typifies the labor of IVD manufacturers to develop low-cost POC instruments that are fast, accurate, and reliable.
Test Menu. To achieve the menu necessary for a POC testing site to perform all the functions of a full-service laboratory, the site would need to have at its disposal technology equivalent to several large desktop or floor-model systems, including a chemistry analyzer, a coagulation analyzer, an immunochemistry analyzer, and a hematology analyzer. Combining all of these functions in a single POC instrument has proven to be technologically challenging. To date, most POC instruments have been designed to perform either a narrow range of tests, such as those for blood gases and other critical-care analytes (I-Stat, Diametrics, AVL); a single specialty test, such as for glucose (Johnson & Johnson Lifescan, Abbott Laboratories, Roche Diagnostics), lipids (Cholestech), drugs (BioSite), coagulation (International Technidyne, PharmaNetics), or cardiac markers (Dade Behring, BioSite); or a limited menu of routine tests (Abaxis, Roche Diagnostics). Such testing platforms are capable only of supplementing—not replacing—the functions of a central laboratory.
The Careside Analyzer offers a broad menu of routine blood tests, currently including 37 FDA-cleared or FDA-exempt tests or test parameters in the three categories of chemistry, electrochemistry, and coagulation. Immunochemistry testing will be added to the system's test menu by the end of this year. (Hematology tests are conducted on a separate device.)
Ease of Sampling. The specimens used in current POC devices are typically microliter volumes of whole blood or urine—a considerable reduction from traditional laboratory tests, which usually require much larger sample volumes. In most cases, the dosing process for such devices requires only semiquantitative transfer of the sample to the cartridge or instrument. Like other such devices, the Careside cartridge uses approximately 80 to 100 µl of whole blood, which the operator transfers into the sample well to a point indicated on the cartridge. The operator does not need to measure the blood sample precisely, because the channels within each cartridge automatically measure and control how much sample is applied to the reagent. The cartridge is sealed once the sample has been applied, thereby protecting the user from exposure to the blood.
Cartridge Technology. For several reasons, most manufacturers of instrumented POC tests have chosen to use a cartridge-based system for unit-dose testing. Such a system enables the manufacturer to preselect and optimize all of the reagents necessary to perform the test, thereby freeing laboratory personnel from responsibility for the error-prone process of preparing test reagents. A cartridge-based system also provides the manufacturer with a place to bar code assay parameters specific to each test, making it simple for technicians to conduct a particular test at any time and in any sequence. A cartridge-based system also offers manufacturers the opportunity to design a number of test operations into the cartridge—rather than into the instrument—thus making the instrument smaller, easier to use, and less costly.
The key enabling technology of the Careside Analyzer is its patented series of cartridge designs. Externally, each cartridge design looks the same: each is molded in polystyrene, measures approximately 2½ x 1½ in., and is designed for one-time use. To avoid error, the cartridge is designed so it can be inserted into the analyzer in only one direction. The size and shape of the cartridge makes it inexpensive to manufacture on an automated assembly line, easy to handle, and yet large enough to accommodate the unique array of microfluidic chambers and channels needed for different tests.
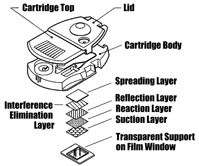
Internally, the Careside series of cartridges includes four distinct designs that make it possible to conduct chemistry, electrochemistry, coagulation, and immunochemistry tests with a single instrument.  The chemistry cartridge is designed for dry, film-based tests and uses a 10 x 12-mm pad of film impregnated with test-specific reagents as its reaction site. The signal from the film is detected from the bottom of the cartridge using reflectance optics. The electrochemistry cartridge performs tests only on whole blood, and employs an ion-specific electrode to measure electrolyte concentrations. The coagulation cartridge contains a miniature cuvette that is filled with sample and on-board liquid reagents during the testing process. Signal detection is accomplished using optical transmission techniques. The immunochemistry cartridge works in the same manner as the coagulation cartridge, except that it requires only one-fifth as much sample volume.
The chemistry cartridge is designed for dry, film-based tests and uses a 10 x 12-mm pad of film impregnated with test-specific reagents as its reaction site. The signal from the film is detected from the bottom of the cartridge using reflectance optics. The electrochemistry cartridge performs tests only on whole blood, and employs an ion-specific electrode to measure electrolyte concentrations. The coagulation cartridge contains a miniature cuvette that is filled with sample and on-board liquid reagents during the testing process. Signal detection is accomplished using optical transmission techniques. The immunochemistry cartridge works in the same manner as the coagulation cartridge, except that it requires only one-fifth as much sample volume.
 Careside's microfluidic cartridges for albumin testing (top) and triglycerides (breakaway diagram, above).
Careside's microfluidic cartridges for albumin testing (top) and triglycerides (breakaway diagram, above).
Each cartridge contains all reagents necessary to perform a particular test. When refrigerated, the cartridges have a shelf life of up to 18 months. The cartridges are loaded into the instrument manually, and can be used directly from the refrigerator, thereby saving the 30 to 60 minutes of warm-up time required by other POC cartridges. To avoid the possibility of condensed water interfering with signal detection, which is a common problem for systems that require refrigerated storage of reagents, the Careside Analyzer warms up the cartridge in a noncondensing environment. When in use, all of the Careside cartridges share certain common features and functions. Chambers and channels molded into the base plate of each cartridge interact with the instrument to ensure proper reagent and specimen temperature equilibration, sample separation, sample metering, sample dispensing, test incubation, and result detection. They control the separation of plasma from cells; meter the amount of specimen required for the test; and facilitate the flow of the blood, serum, or plasma specimen onto dry reagents packaged in the cartridge.
By employing varied cartridge designs that make use of different detection technologies—instead of several separate analyzers—the Careside approach enables the POC testing site to reduce the number of instruments it must purchase. In turn, the approach makes operation of the tests easier, and enables Careside to select from several technology platforms when developing a new test. This approach is unique among the POC testing devices currently on the market.
User Interface. If POC testing is to be a commercial and technical success, the POC device must guide the user through the testing process and reduce the skill level necessary to perform and document a test. To date, most POC device manufacturers have concentrated their efforts on designing instruments capable of displaying and storing final test results. They have largely ignored the many other functions commonly performed by clinical laboratory systems, such as collection of patient demographic information, test requisition, data retrieval, and quality control performance and documentation. On most current POC devices, the user interface is limited to preprogrammed functions on a microprocessor, accepts only numeric inputs from a membrane keypad, and displays results using a one- or two-line LCD.
 A touch-screen interface helps reduce instrument size.
A touch-screen interface helps reduce instrument size.
In the Careside Analyzer, more than 700,000 lines of custom software code have been programmed in an embedded PC to simplify and automate many steps of the testing process previously left to the discretion of POC testing personnel. Commands are entered on a 6.8-in. touch screen. Under the setup menu, users can customize features of the user interface to meet the needs of their specific location. Selectable features include languages, security password protection for up to 1000 users, date and time logging, custom reports, patient demographic information, tests to be performed, custom test panels, reference intervals, alert ranges, quality control parameters, and cartridge lot numbers.
 A built-in thermal printer allows reports to be output on command.
A built-in thermal printer allows reports to be output on command.
All of the settings may be transferred to and from a master disk via the analyzer's internal disk drive. Software versions are also automatically upgraded using the disk drive. Help screens and error messages make operation of the instrument simple. Nontechnical personnel can be trained on the operation of the instrument in less than 30 minutes. The Careside Analyzer is designed to perform testing on one patient at a time. Up to 1000 patient reports are stored on the instrument and can be searched and printed on command.
 Operating instructions and test documentation are conveyed via a bar code reader.
Operating instructions and test documentation are conveyed via a bar code reader.
Testing Documentation. POC devices using unit-dose cartridges need a way of communicating to the instrument what test is being performed as well as its lot number, expiration date, and calibration information. Such information can be input into the instrument by using an EPROM, magnetic strip, bar code label, or keypad. The Careside Analyzer uses information encrypted in the cartridge bar code. As each test is completed, this information becomes part of the system's CLIA documentation. Because each cartridge contains an identifying bar code that is read by the instrument, cartridges may be inserted in any order. The analyzer checks that the ordered tests match the cartridges inserted into the device.
Quality Control. The Careside Analyzer's software includes an extensive automated quality control application not found in any other POC device. The software stores and interprets the quality control data generated using embedded and external electronic quality control checks. Traditional wet quality control testing using control solutions and test cartridges can also be run on the instrument. Reusable quality control test cartridges enable the user to automatically perform electronic quality control for reflectance-, transmission-, or electrochemistry-based tests. These cartridges reduce the need to perform traditional wet quality control testing and the cost of quality control activity. After testing, quality control data are plotted on graphical charts. If quality control has not been performed according to the user-defined schedule or the results are out of limits, the discrepancy is flagged. The instrument automatically locks out the user if quality control results exceed the limits selected by the laboratory director. Only an individual with a supervisor pass code can unlock the instrument and approve continued testing.
Sample Preparation. One of the major challenges facing POC devices is specimen preparation. Manufacturers seeking to automate this part of POC testing must decide whether their instrument will use whole blood or plasma, whether sample preparation must be done outside the device or can be built-in, and whether their device should be designed to perform batch or single-test runs. In each of these cases, whatever the manufacturer decides will require trade-offs in other aspects of the instrument's design.
 The rotating platter positions cartridges for testing and eliminates the need to use external centrifugation.
The rotating platter positions cartridges for testing and eliminates the need to use external centrifugation.
The Careside Analyzer contains a rotating platter that holds up to six cartridges, and is automatically balanced for any number of randomly inserted cartridges. The platter moves cartridges to various positions during performance of a test, and retains the cartridges during processing and data collection. It applies centrifugal forces to transfer the specimen from the specimen well to other parts of the test cartridge and separates plasma from blood cells, thereby eliminating the need for external centrifugation. Because of the versatility of its cartridge designs and instrument performance, the Careside Analyzer can accommodate either whole blood or plasma tests.
Sample-Transfer Mechanism. The sample-transfer mechanism provides the force needed to move a metered amount of sample in the cartridge onto the reagent stack. The mechanism moves a plunger down onto a flexible seal on the cartridge lid while simultaneously sealing the cartridge vent. The downward force in conjunction with the design of the cartridge creates pressure that moves the metered sample onto the reagent stack.
 A view of the platter's underside reveals portions of the analyzer's optics systems.
A view of the platter's underside reveals portions of the analyzer's optics systems.
Optics and Analysis. The rotating platter moves cartridges across the instrument's various optics systems to enable detection of results. The platter incorporates black-and-white optical standards used by the system's main optics to calibrate readings. For reflectance and transmission readings, the optics collect data continuously over 2 to 6 minutes. These data are then averaged and analyzed, providing a highly accurate and precise final result. For electrochemistry readings, the cartridge stops at a detection station and makes contact with a set of pogo pins designed to measure the voltage difference in ion-specific electrodes built into the cartridge.
HOW IT WORKS
The system's custom user interface provides sequential instructions that prompt the user to begin by entering their four-digit PIN number, patient, physician, and test information via the touch screen. The operator selects from refrigerated inventory one or more test cartridges corresponding to the tests ordered by the attending healthcare provider. Most cartridges contain one test, but some contain up to three tests. The analyzer is currently capable of conducting a maximum of eight tests per patient in a 12-minute test cycle. When development of additional multiplexed test cartridges is completed, the system will be able to handle up to 13 tests in a single 12-minute test cycle.
 After adding a sample, the cartridge is sealed to protect the user from exposure to blood.
After adding a sample, the cartridge is sealed to protect the user from exposure to blood.
You May Also Like



