Preparing for Implantable Electronics of the Future
April 11, 2013
There is a push towards new kinds of implantable electronics packaging said Jason Clevenger, PhD, from Exponent (Menlo Park) at BIOMEDevice Boston on April 10. The trend is driven in part by the miniaturization of components and power sources, which has led to new applications such as implantable neurostimulators and personal health monitoring devices that can track conditions such as glaucoma and diabetes as well as help monitor medication compliance.
The 1960s saw a milestone in implant packaging with the debut of titanium-alloy packaging that was initially introduced by the military. Long used for implantable devices such as pacemakers, this packaging type may be "tried and true," but is bulky. In the 1990s, glass / metal bonding and ceramic / metal bonding debuted, which have been used for applications such as cochlear implants and artificial retina implants.
|
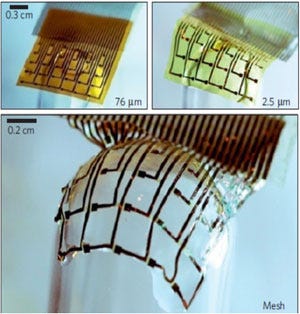
Dissolvable electronic films could be used for implantable applications and dissolve in a programmable time frame. |
The packaging for implantable electronics will draw from both materials science breakthroughs and advances in electronics manufacturing, Clevenger said.
Polymers will likely be used increasingly for implantable electronics packaging. Traditionally, however, they have been difficult to employ for many implant applications--especially over the long term--because they are porous and permeable to water and can be expensive to process. As such, polymers have been used for low-voltage applications.
One potential candidate polymeric material for long-term implant applications is liquid crystal polymers, which have molecules that can be mutually aligned and organized. The material has a high molecular order and offers a good strength and chemical barrier profiles. In tests, LCP has bested both polyimide and parylene.
Devices that are based on MEMS material platforms are another candidate for implants. Breakthroughs in chip encapsulation are poised to lead to miniaturized biomimetic packaging for implantable electronics.
LCP polymers and MEMs materials seem to be especially suited for conformal, lightweight implants that are used long term.
Another strategy is to layer polymers together in a packaging to achieve substantially better barrier properties than individual materials could offer alone. Yet another is to explore the use of dissolvable electronic films, which, although not a traditional packaging material, offer a promising bioelectronic interface for many implantable applications.
To conclude, Clevenger advised that design engineers should experiment with different types of packaging for implantable electronics while using robust Failure Mode Effects Analysis strategies.
Brian Buntz is the editor-in-chief of MPMN. Follow him on Twitter at @brian_buntz.
About the Author(s)
You May Also Like