Abbott Wins FDA Approval for New and Improved IOL
July 18, 2016
The Tecnis Symfony IOL is the first intraocular lens able to allow cataract patients to have extended depth-of-focus, according to FDA.
Chris Newmarker
FDA recently announced approval of an intraocular lens to help cataract sufferers improve the sharpness of their vision at near, intermediate, and far distances--providing a level of eyesight improvement not seen in other IOL lenses.
The approval of Abbott's Tecnis Symfony IOL could be good news for fifth of Americans expected to develop cataracts by age 65, potentially requiring cataract surgery to replace the clouded natural lens with an IOL.
"While IOLs have been the mainstay of cataract treatment for many years, we continue to see advances in the technology," said Malvina Eydelman, MD, director of the Division of Ophthalmic and Ear, Nose and Throat Devices in the FDA's Center for Devices and Radiological Health. "The Tecnis Symfony Extended Range of Vision IOL provides a new option for patients that may result in better vision across a broader range of distances."
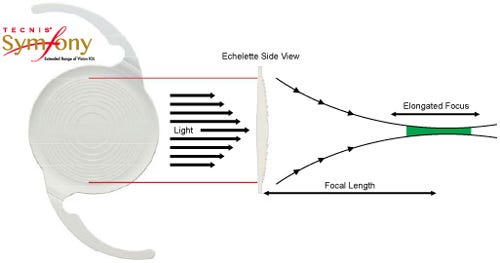
The lens includes a proprietary diffractive echelette design feature. The design feature's light diffraction pattern elongates the focus of the eye, extending vision range.
FDA approval came after the agency reviewed results of a randomized clinical trial that compared 148 cataract patients implanted with the Tecnis Symfony IOL with151 cataract patients with a monofocal IOL. More than three-fourths of the patients with the Tecnis Symfony IOL had good vision without glasses at intermediate distances, versus 34% for the monofocal IOL group. When it came to near distances, the patients implanted with the Tecnis Symfony IOL saw two additional, progressively smaller lines on a standard eye chart. Distance vision was comparable for both groups.
Chris Newmarker is senior editor of Qmed. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like