Implantable Wireless Device Monitors, Treats Bladder Dysfunction
September 23, 2013
Bladder dysfunction, including urinary incontinence and overactive bladder, is estimated to affect some 25 million people in the United States. Currently, bladder dysfunction and urinary incontinence resulting from lower urinary tract dysfunction are diagnosed in a laboratory setting by inserting a catheter through the urethra into the bladder and performing retrograde filling of the bladder at a higher than normal rate, usually with room-temperature water or saline. While this test is being performed, a doctor or nurse observes the patient and asks, "Do you have to go?"
A very artificial procedure, this test is unable to reproduce the patient's symptoms in a surprisingly high percentage of patients--up to 50% in some populations, according to Margot Damaser, staff member in the department of biomedical engineering at the Cleveland Clinic's Lerner Research Institute and associate professor in the Cleveland Clinic Lerner College of Medicine (Cleveland). On September 27 at MEDevice San Diego, Damaser will discuss an alternative method for diagnosing bladder dysfunction in a presentation titled "Technology for Wireless Catheter-Free Sensing of Bladder Pressure." In the following Q&A, she addresses bladder dysfunction and highlights her work in developing a new wireless implantable device for monitoring this condition.
MPMN: Please describe your wireless catheter-free device for sensing bladder pressure. What medical need were you addressing in developing this device?
|
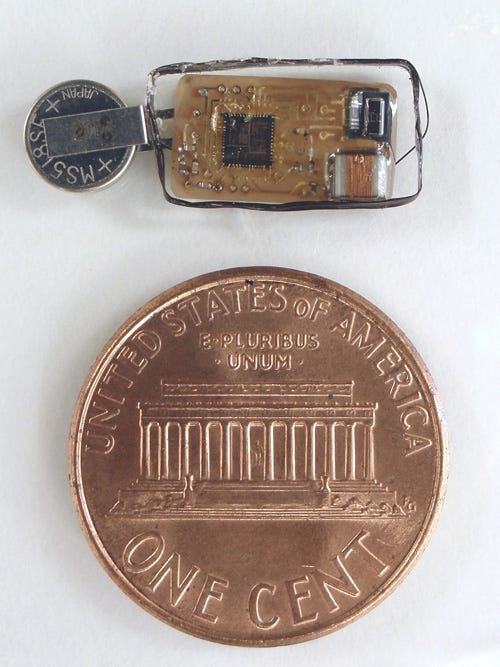
A new wireless catheter-free implantable device can perform short-term diagnostics in the bladder and also be used to treat chronic conditions. |
Damaser: Our wireless catheter-free device has two applications. In the first, it is used to perform short-term diagnostics, while in the other, it is used as a chronic implantable device. Both applications rely on the same wireless technology.
Many years ago, I started asking myself as an engineer why we don't have a Holter monitor for the bladder--a wireless catheter-free device that could be implanted and that the patient can go home with. While there is a Holter monitor for the heart, there is no such device for the bladder. The advantage of using wireless technology in this application is clear: No one is going to put up with a diagnostic device in which a wire comes out of the bladder.
As we pursued the development of this technology, it became clear that our device could be used to perform another function: providing feedback to an electrical stimulation system. At present, neuromodulation systems can be implanted to treat people suffering from incontinence, but the electrical stimulation is constantly turned on. Consequently, the nervous system habituates to it so that in every patient, it has to be readjusted about every six months. Existing research data suggest that if electrical stimulation could be turned on only when needed, if there is some kind of closed-loop control--from a pressure monitor in the bladder, for example--the stimulation device would save power and energy, and it would also be much more effective because the nervous system would not habituate to it. Since our device can perform this role, it has the potential to change both how incontinence is diagnosed and how it's treated.
MPMN: What about the wireless technology itself? How does it work and what does it do?
Damaser: First of all, we had to come up with a wireless integrated circuit that requires very low power. Designed to reduce power consumption as much as possible, our wireless implementation fits this bill. The transmitter is switched on only when needed. When there is no bladder activity, there is no transmission, and when there is bladder activity--as measured by the rudimentary smarts inside the device--data are transmitted. As a result, the transmitter--the power-hungry portion of the device--is effectively turned off about 99% of the time. That's part of what makes our device different.
As you might imagine when thinking of wireless transmission, the challenging part of our transmission scheme is receiving the signal reliably. To ensure the system's reliability, we had to design a custom receiver that quickly locks on to the transmitted signal because the receiver never knows when it's coming. That was a challenge that I believe we have met. Our receiver can currently pick up a signal transmitted from about 20 cm away, which is sufficient for an external receiver and avoids the need for an implanted receiver.
We overcame this challenge by using near-field inductive communications, which allows for a relatively low carrier frequency and enables less power to be transmitted through the body. Using this method, we were able to achieve the transmission distance we need for the device to work reliably. The receiver locks on using digital signaling between the transmitter and the receiver. The implanted transmitter sends out a tone, which the receiver can lock onto to receive the data.
MPMN: What about the transmitter? How is it configured?
Damaser: Inserted into the bladder using a cystoscope, the implantable device is the size of an oversized pill. This pill contains the transmitter, a sensor, a battery, some smarts, and a coil for transmission, and it's all packaged within a device measuring about 15 x 5 mm. To insert the implant in patients suffering from chronic bladder dysfunction, the physician makes a small incision in the urothelium inside the bladder lumen and slides the device under the lining between the urothelium and the muscle. The device must not make contact with the urine stream because anything in contact with the urine stream for a long time will cause stone development.
MPMN: How do you charge battery once the device is inserted inside the bladder?
Damaser: Battery charging occurs wirelessly. The power usage is designed so that the battery lasts for 72 hours. The idea is that a patient can recharge it overnight while the device is still functioning to provide feedback to an electrical stimulation system. But if the patient uses a recharger placed under or near the bed or under the mattress, for example, the battery should be able to recharge sufficiently overnight. However, if the patient skips a night, the device will continue to function properly.
Recharging works along similar lines as in the wireless rechargers found in smartphones or consumer products such as battery-operated toothbrushes. The external device generates a magnetic field, which is coupled to the implanted device--again over a distance of 20 cm. The implanted device also contains a custom battery recharger circuit in which the transmission coil is used to receive the current from the magnetic field and translate it into power for the device.
MPMN: What considerations have you given to data security?
Damaser: The only data security measure we have taken to date is that the bladder device relies on very near-field communications and very low power. Thus, reception of the signal from outside is quite challenging, and eavesdropping is really only practical from very nearby. While eavesdropping could take place from farther away than the device's 20-cm transmission range, doing so would, in essence, require the electronic equivalent of huge headphones drawing large amounts of power. But even then, the eavesdropper would have to be within 10 m of the patient.
Because our device is investigational at this time and is only beginning to be used in animal studies, we haven't put a lot of effort into more specific data security measures. However, encryption is a possibility--one that I think is an obvious choice when we get to that point.
MPMN: Following the animal studies, do you envision that your technology will be marketed anytime in the near future?
Damaser: Yes. The technology that we have developed certainly could be marketed once its efficacy has been proved in an animal study. At the same time, we're beginning to partner with a couple of companies that could make marketable prototypes. The prototypes that we manufacture for laboratory testing are neither marketable nor mass-producible, but the technology would be the same as in marketable prototypes. To implement the commercialization and eventual marketing of the technology, we are working with CCF Innovations, the technology commercialization arm of the Cleveland Clinic; the Veterans Administration Technology Transfer Program; and the technology transfer department at Case Western Reserve University's School of Engineering.
MPMN: Do you think that the platform that you're developing now could be expanded to serve other implantable medical device applications beyond the bladder?
Damaser: Absolutely. One reason we started with the bladder is that this is my area of expertise, and I have been dying to develop a technology for patients that I have seen in other studies. Moreover, developing a device to monitor bladder activity is a particularly challenging application. To implant a device in the bladder, we have to be able to insert it through the very narrow urethra, and if we can achieve that, we can certainly insert it in other body cavities, including the rectum, colon, or even the stomach.
Thus, there is a great deal of interest in our device from other fields, even if we limit it to pressure-sensing technology. In addition to the colorectal and gastrointestinal areas, we have encountered interest from those working in the cardiovascular field. The same technology could be used to sense other physiological signals as well. Thus, I think there are myriad other suitable applications, and I think that we've begun by tackling one of the more difficult ones. Once we see this one through, the other applications should come fairly quickly.
Bob Michaels is managing editor of Medical Product Manufacturing News.
About the Author(s)
You May Also Like