Examining Potential EMI between Medical Devices and Electronic Security Systems
Medical Device & Diagnostic Industry MagazineMDDI Article IndexOriginally Published January 2000EMIGiven safety concerns and the complex nature of electromagnetic interference with medical devices, regulators are taking a cautious approach.
January 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Originally Published January 2000
Given safety concerns and the complex nature of electromagnetic interference with medical devices, regulators are taking a cautious approach.
Several recent reports have raised concerns about the potential risks for medical device users from electromagnetic interference (EMI) with the normal operation of such devices by certain types of electronic security systems, including electronic article surveillance (EAS) systems and metal detectors. While the number of reported significant patient injuries from EMI with EAS systems and metal detectors is low, the information to date suggests that the electromagnetic energy emissions from EAS systems and metal detectors can interact with some critical medical devices.
Although the full extent of the interactions on specific medical devices is not known, there does not appear to be a major public health concern at this time. However, with the increasing complexity and portability of medical devices and the proliferation of EAS systems and metal detectors, the FDA's Center for Devices and Radiological Health (CDRH) has become concerned about the potential for EMI between these technologies and medical devices.
EAS antitheft systems have become widely used in commercial establishments, and metal detectors are used for security in many buildings. Both of these kinds of electronic security systems emit electromagnetic energy to detect the presence of a special tag (EAS systems) or metallic materials (metal detectors) (Figure 1). With the limited information currently available, CDRH has sought to engage medical device and security system manufacturers in ways that will address medical device user concerns while not unduly alarming device users or clinicians.
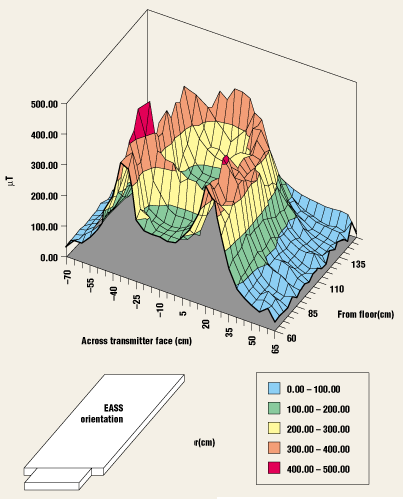
Figure 1. Measurement of the electromagnetic fields from a typical electronic article surveillance system. The pulsed magnetic vertical plane is 6 cm from the transmitter pylon face. (Source: Engineering Characterization of Common Electronic Article Surveillance Systems Electromagnetic Fields by Jon Casamento).
CDRH has taken an active role in calling attention to the potential risk to medical device users. The agency has performed laboratory research, raised public awareness, and provided a scientific forum to address these concerns. For example, on September 24, 1998, CDRH organized a discussion of its concerns about medical device EMI from EAS systems and metal detectors before the Technical Electronic Product Radiation Safety Standard Committee (TEPRSSC), an advisory committee established under the Radiation Control for Health and Safety Act of 1968. At this public meeting, CDRH scientists and a physician outlined CDRH's current information regarding these EMI interactions, including: an analysis of medical device reporting (MDR) network reports, a review of the technical literature, and the results of laboratory measurements performed by CDRH. Manufacturers of medical devices and EAS systems and metal detectors also presented their perspectives. In addition, several leading cardiologists presented their clinical impressions of the significance of medical device EMI from these systems.
As a result of the information presented, the TEPRSSC agreed with CDRH's plan to inform physicians about the potential for EMI from EAS systems and metal detectors.1 Further, CDRH is working with the Association for the Advancement of Medical Instrumentation (AAMI) and the American Society for Testing and Materials (ASTM) to help formulate standards for medical devices (e.g., cardiac pacemakers and implantable cardiac defibrillators [ICDs]) and metal detector systems, respectively, to address EMI with both the medical devices and the security system emitters.
CDRH has analyzed more than 50 MDR reports dating back to 1987 for suspected incidents of security system EMI with medical devices. Several reports contain information about serious patient consequences related to EMI from EAS systems, metal detector systems, or security systems. The largest group of reports (33) involved moderate to severe patient consequences from EMI with cardiac pacemakers and ICD devices. Twenty reports involved EMI with implanted spinal cord stimulators used for relief of chronic pain. Most of these adverse interactions were considered as moderate in level of severity. Examples of the most significant MDR reports for several medical devices are summarized below.
A pacemaker patient reportedly lost consciousness when standing for approximately 2 minutes near an EAS system tower (MDR 573141).
In four separate incident reports, patient ICDs were reprogrammed into the inactive mode after the patient passed through, or was hand-scanned by, a metal detector (MDR 806666, MDR 858835, MDR 212215 1997 00106, MDR 2124215 1997 00229).
A pacemaker patient's pulse dropped from 70 down to 31 beats per minute while being interrogated with a metal detector in an airport (MDR 358472).
An inappropriate firing of an ICD occurred when a patient leaned against an EAS system pylon in a grocery store (MDR MW1006883).
An overinfusion of drugs occurred after a patient had passed through a metal detector; the device manufacturer reported that the infusion pump was functioning properly when tested after the incident (MDR 232087). Dialysis was later required to remove the excess drugs.
While near a security system, an implanted spinal cord stimulator patient experienced a strong shock, followed by sporadic shocks that resulted in unconsciousness and hospitalization (MDR 6000033 1997 00079).
While the numbers of MDR reports may be relatively small, the types of interactions reported serve as a valuable indicator of potential problems. In many cases, manifestations of EMI effects appear to be only intermittent or momentary. As a result, it can be difficult to associate the adverse interaction with a specific interference phenomenon or known source of EMI. With interference from an EAS system, for example, patients may experience some device interaction while they are within the EAS system field. The noticeable effects of the EMI may quickly diminish once the patient has exited the system. In many reported EMI cases, the effects appear to result in immediate patient symptoms such as a change in heart rate or overstimulation to nerve tissue. However, in some cases a patient might not immediately associate the adverse interaction with the exposure, yet the possibility of suffering serious consequences remains (as in the cases of drug overinfusion or reversion of an ICD to monitor mode mentioned above). Unlike interference from medical device users' personal handheld transmitters, such as cellular phones (where the user is aware of the EMI source and, for the most part, is voluntarily exposed), security systems are widespread and deliberately placed in locations that are difficult to avoid. In some cases, an EAS system may be hidden and patients may not even be aware of the electromagnetic exposure. In view of the variable and complex nature of EMI disruptions of medical devices, a cautious approach toward addressing concerns for medical device safety and effectiveness is warranted.
REPORTS IN TECHNICAL AND MEDICAL LITERATURE
Potential EMI with cardiac pacemakers from a variety of electromagnetic sources is widely known in the clinical community and is addressed by manufacturers in device design, labeling, and education. However, only a few studies have targeted EMI from EAS systems, and even fewer have addressed metal detector systems. Large multicenter studies, like those performed for other EMI concerns such as cellular telephones, seem to be absent.2 Nevertheless, there have been a few case studies reported in the medical literature that involve EMI with ICDs and spinal cord stimulators. For example, McIvor reported on one such incident in which an ICD patient leaned up against an EAS system and experienced a defibrillation shock.3 Mathew et al. have also reported an incident linking an ICD output shock with an EAS system.4 In addition to the ICD reports, Eisenberg and Waisbrod reported on a serious injury to a patient with an implanted spinal cord stimulator exposed to an EAS system.5
McIvor also performed a study with 25 ICD patients and 50 pacemaker patients that were exposed to six different EAS systems in a systematic approach.6 While no interactions were seen with the ICD patients in this study, nearly all of the pacemaker patients experienced some interaction with one or more of the EAS systems. The interactions reported varied depending upon the pacemaker, the testing protocol, and the type of EAS system. Exposure to one type of EAS system (pulsed magnetic) revealed signs of EMI in 48 out of 50 pacemaker patients, with some patients experiencing dizziness. McIvor characterized the interactions in four main types, some with significant clinical implications. However, none of the pacemakers or ICDs in this study were reprogrammed by the interactions reported by McIvor.
Wilke has also reported on interference with implanted pacemakers by EAS systems.7 His study indicated that the pacemakers in 7 out of 53 patients experienced some type of interference with an EAS system that had higher electromagnetic fields associated with it. As a result of this study, Wilke suggests that pacemaker patients should avoid coming close to an EAS system for any length of time.
Medical Device
Continuous Wave Magnetic | Pulsed Magnetic | Swept Radio Frequency | Microwave | |
|---|---|---|---|---|
Cardiac pacemaker | 54%(29/54) | 44%(24/54) | 12%(4/33) | 0%(0/5) |
Implanted cardiac defibrillator | 0%(0/1) | 0%(0/1) | — | — |
Table I. In vitro EAS system interactions with medical devices from published studies.
In addition to the studies and reports with patients, there are several reports of work performed in vitro using ICDs and pacemakers.8–10 The results of these studies suggest that lower-frequency and pulsed-type EAS systems might interfere more with implanted medical devices than the swept radio-frequency (RF) or microwave systems (Table I). Unfortunately, there is a dearth of such studies for metal detectors.
Taken as a whole, some common threads emerge from the published work that are consistent with CDRH's experience with medical device EMI. For instance, many medical devices are designed to sense and react with physiological signals, which are usually low frequency (e.g., from 0.5 to about 10 Hz) and modulated. CDRH's experience with EMI problems in other devices (e.g., apnea monitors) indicates that external electromagnetic signals, with amplitude modulation falling within the band pass of the physiological sig-nal being measured, would likely be where interactions would occur. Indeed, the IEC 60601-1-2 standard, the most prominent electromagnetic compatibility standard for medical electrical equipment, currently requires that radiated immunity testing be performed with exposure to a signal modulated within the most significant band pass of the device or with a default modulation.11 Therefore, potentially offending outside signals with modulation characteristics close to the physiological signals being measured by a medical device are much more likely to interfere than other signals. This is not to say that medical devices exposed to electromagnetic fields with no low-frequency amplitude modulation would be immune to EMI. On the contrary, the in vitro results in the table clearly suggest that the swept RF EAS system caused EMI in a small, but not insignificant, portion of devices.
FDA LETTER TO PHYSICIANS
Following the TEPRSSC meeting, CDRH sent a letter to cardiologists, neurologists, and other clinicians to provide basic information about concerns for medical device EMI from EAS systems and metal detectors and offer a few suggestions intended to minimize risk. This letter contains a brief synopsis of the FDA MDR network incident reports relating to these interactions. The letter points out that the number of reported significant incidents is very low, and that CDRH is working with the device manufacturers and EAS system and metal detector manufacturers to develop solutions. It also contains some simple precautions for patients who use electronic medical devices that may be exposed to EAS systems or metal detectors. Among these suggestions are:
Be aware that EAS systems may be hidden and not readily visible.
Avoid staying near an EAS system or metal detector any longer than necessary; avoid leaning on these systems.
If a security check with handheld metal detectors is required, alert the security personnel about any implanted or patient-connected medical device and request minimal exposure, or, if possible, an alternative form of search.
If any device malfunction is noted, users should make this known to their physician and/or the device manufacturer. In cases of serious interactions and health consequences, manufacturers must report such incidents to FDA. The device user or medical practitioner can also report the incident directly to FDA under the MedWatch program (telephone 800-FDA-1088).
Harthorne and others have suggested that the brief time for normal passage through an EAS system or metal detector gate would tend to minimize the chances for clinically significant EMI with medical devices.12 In many instances, this may well be the case, and might account for the relatively small number of reports considering the large volume of patients exposed to these systems. However, there are many situations where passage through the security system or exposure to handheld detectors occurs over an extended time period. For example, delays at a busy airport security station may keep a person within the electromagnetic field longer than just a few seconds. Alternatively, security system configurations that place an EAS system near a cashier or workstation are not uncommon. Such configurations appear likely to expose a person to the electromagnetic field for longer periods of time. In addition, exposures to handheld metal detectors are usually of short duration, but there are incident reports involving these products where the exposure appears to have been longer.
If time constraints on exposure to security systems is not always practical, then an understanding of the electromagnetic field characteristics around the EAS system or metal detector could provide a predictor of the potential for EMI. A suggestion for this has been made by McIvor et al.6 In their paper, calculations were made of the induced voltages onto a pacemaker lead system loop of 200 cm2 using measurements of the electromagnetic fields of the EAS system used in the study.
The paper explains that some of these induced voltage values calculated from EAS system measurements fall outside the specifications for normal and defined pacemaker operation from the European pacemaker standard EN 50061/A1.13 The result of some of the larger EAS-system- induced voltages might, under circumstances such as the patient remaining in the EAS-system electromagnetic fields for an extended period of time, lead to pacemaker malfunction and possible patient risk.
Concern has also been noted because the emissions (carrier frequency or modulations) from different types of electronic security systems can fall within the physiological passband of several kinds of ambulatory medical devices. However, the scarcity of public information about the characteristics of the electromagnetic fields emitted from these systems makes it difficult to evaluate the potential susceptibility of medical devices to these fields.
ELECTROMAGNETIC FIELD MEASUREMENTS
Because of the paucity of information on the electromagnetic fields emitted by EAS systems, CDRH has measured these fields around a number of sample EAS systems (Figure 1 illustrates one of these measurements).14 A summary of the results were presented at the TEPRSSC meeting. The CDRH measurements did not encompass all of the types of EAS systems, but did include the following:
An extra-low-frequency (ELF) magnetic continuous wave (CW) system operating at 219 Hz.
A voice-frequency magnetic CW system operating at 535.7 Hz.
Three low-frequency pulsed magnetic systems operating at 58 KHz.
Three frequency-modulated (FM) swept RF systems operating between 1.8 and 2.1 MHz or 7.2 and 9 MHz.
These measurements showed that the electromagnetic fields around the transmitting EAS system pylon vary spatially from top to bottom, side to side, and going away from the pylon. The electromagnetic fields also vary depending on the specific system design and whether the EAS system is used with pairs of pylons (transmitter and receiver) or a single pylon. For example, the measurements revealed that the single-pylon setup showed broader field strength patterns than the patterns around the paired pylons. In addition, measurements of the pulsed magnetic systems at a reference height of 130 cm, and a distance of 36 cm from the transmitter, revealed remarkably consistent field strengths of about 61–65 µT, even though one system was measured with the pylons separated more than 1 m further than the other systems. The highest fields were measured from the ELF magnetic EAS system (122 µT), whereas the swept RF systems were measured at 1 µT or lower.
FUTURE DIRECTIONS
Unfortunately, only a few studies have been published on EAS systems and metal detector interference with a few critical devices such as cardiac pacemakers and ICDs. These studies appear inconsistent about the significance of such interference. In addition, it is not clear whether the types of security systems used in the published studies represent the entire range of technologies in use (e.g., certain types of the lower-frequency magnetic field EAS systems typically used in libraries seem to be unrepresented). Thus, studies with broader objectives that cover the range of security systems need to be mounted to examine both the medical device susceptibility and the clinical relevance of any device-related EMI in terms of effects on the patient. Such an effort should include all potentially susceptible medical device classes, such as spinal cord stimulators, for a variety of malfunctions potentially due to EMI with security systems. Additionally, manufacturers of both the medical devices and the electromagnetic field source products (EAS systems and metal detectors) should work together to minimize the risk for EMI through better communications, design, and testing.
With the pace of technology and the cost factors relating to healthcare both increasing, it can be expected that ever-larger numbers of medical devices will become more complex, with more capabilities and increasing portability. It seems likely then that more medical devices will come into the proximity of EAS systems and metal detector systems. At the same time, the security industry is rapidly advancing, which is driving the need for consistent information about the electromagnetic fields generated by these systems. In addition, there is the need to develop voluntary medical device immunity standards and EAS system and metal detector emission standards to address EMI among these products. Such standards should have consistent test methods reflective of the electromagnetic fields encountered by medical devices. These test methods should be reproducible using readily available and reasonably affordable instrumentation.
Physicians, patients, security system users, manufacturers, and the public all have a stake in obtaining and using the information about the potential for medical device EMI. Since medical devices span a wide range of functions and configurations, there is a great need to evaluate the potential for EMI in a range of devices. The most immediate concern lies with the critical life-supporting and life-sustaining devices, such as cardiac pacemakers. However, other vital medical devices have been affected (e.g., implanted spinal cord stimulators) or could be affected (e.g., portable respirators, infusion pumps, and monitoring systems) by EMI when passing through an EAS system or metal detector.
CONCLUSIONS
With the information gathered by CDRH from MDR reports, clinical and technical literature, and laboratory measurements, there is basis for concern that the normal functioning of some medical devices may be interfered with by EAS systems or metal detectors. This concern is mitigated by the low numbers of documented cases of EMI in view of the estimated large numbers of exposures.
Although it is not considered a major public health problem at the moment, the potential serious health consequences for medical device users and the potential susceptibility of a number of vital medical devices warrant a cautious approach to examining the EMI between EAS systems and metal detectors and medical devices. EAS systems and metal detectors certainly benefit the public in an age of heightened security awareness, but this must be balanced with the potential for EMI with critical medical devices. Medical device manufacturers, EAS system and metal detector manufacturers, and industry regulators must work together to formulate ways to address these EMI concerns so that device users, clinicians, and the public are not unduly alarmed. Voluntary performance standards and equipment labeling can play an important role in establishing medical device EMI immunity and setting reasonable limits on the electromagnetic source emissions from EAS systems and metal detectors.
REFERENCES
1. "Important Information on Anti-Theft and Metal Detector Systems and Pacemakers, ICDs, and Spinal Cord Stimulators," Center for Devices and Radiological Health Letter to Cardiologists, Neurologists, Cardiac Surgeons, Neurosurgeons, and Emergency Physicians, September 28, 1998.
2. DL Hayes et al., "Interference with Cardiac Pacemakers by Cellular Telephone," New England Journal of Medicine 336 (May 22, 1997): 1473–1479.
3. ME McIvor, "Environmental Electromagnetic Interference from Electronic Article Surveillance Devices: Interactions with an ICD," PACE 18 (1995): 2229–2230.
4. P Mathew et al., "Interaction between Electronic Article Surveillance Systems and Implantable Defibrillators: Insights from a Fourth Generation ICD," PACE 20 (1997): 2857–2859.
5. E Eisenberg and H. Waisbrod, "Spinal Cord Stimulator Activation by Anti-Theft Device: Case Report," Journal of Neurosurgery 87 (December 1997): 961–962.
6. ME McIvor et al., "Study of Pacemaker and Implantable Cardioverter Defibrillator Triggering by Electronic Article Surveillance Devices (SPICED TEAS)," PACE 21 (1998): 1847–1861.
7. A Wilke et al., "Interactions between Pacemakers and Security Systems," PACE 21 (1998): 1784–1788.
8. E Lucas, D Johnson, and B McElroy, "The Effects of Electronic Article Surveillance Systems on Permanent Cardiac Pacemakers: An In Vitro Study," PACE 17, part 2 (1994): 2021–2026.
9. B Dodinot, J Godenir, and A Costa, "Electronic Article Surveillance: A Possible Danger for Pacemaker Patients," PACE 16, part 1 (1993): 46–53.
10. KS Tan and I Hinberg, "Can Electronic Article Surveillance Systems Affect Implantable Cardiac Pacemakers and Defibrillators?" PACE 21 (1998): 960.
11. International Electrotechnical Commission, Medical Electrical Equipment—Part 1: General Requirements for Safety—2: Collateral Standard: Electromagnetic Compatibility—Requirements and Tests, IEC 60601-1-2 (Geneva: International Electrotechnical Commission, April 1993).
12. JW Harthorne, "Theft Deterrent Systems: A Threat for Medical Device Recipients or an Industry Cat Fight?," PACE 21 (1998): 1845–1846.
13. Safety of Implantable Cardiac Pacemakers, EN 50061:1988/A1:1995 E (Geneva: CENELEC, August 1995).
14.J Casamento, "Characterizing Electromagnetic Fields of Common Electronic Article Surveillance Systems," Compliance Engineering 16 (September/October 1999), 42–52.
Donald Witters is a biomedical engineer in the Office of Science and Technology at the FDA Center for Devices and Radiological Health (Rockville, MD). He is chairman of the CDRH EMC work group and has performed laboratory research, published papers, presented findings, and organized conferences on medical device EMC. The author wishes to thank the following CDRH staff for providing valuable information and insight during the preparation of this article: Stuart Portnoy, MD; Jon Casamento; and Nancy Pressly.
Return to the MDDI January table of contents | Return to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
You May Also Like


