Philips Gets App-Based Ultrasound Into More Places
October 17, 2016
Introduced last year, the Lumify will now include cardiac, abdominal, OB/GYN, and trauma exam pre-sets.
Nancy Crotti
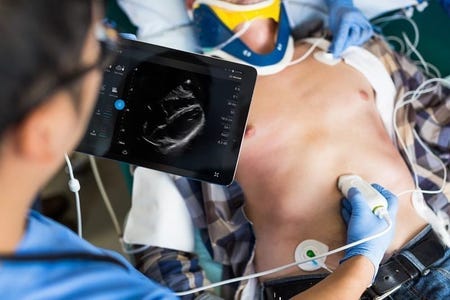 Royal Philips has won FDA clearance to use a cardiac transducer with its app-based Lumify ultrasound wand, and as with previous indications of Lumify it will be available by subscription.
Royal Philips has won FDA clearance to use a cardiac transducer with its app-based Lumify ultrasound wand, and as with previous indications of Lumify it will be available by subscription.
Lumify, which runs off an app on a tablet, will now include cardiac, abdominal including lung, OB/GYN, and trauma exam pre-sets, according to a statement by the company.The pocket-sized and lightweight S4-1 transducer also offers advanced sensitivity and high-resolution 2D image quality. It's Philips' first ultrasound device for ambulatory use. It was tested for reliability in emergency, critical care and ambulance use.
The S4-1 transducer (plus cable) weighs 152 g and is smaller than a smartphone. Lumify also uses cloud-enabled technology to connect with PACS, shared networks and system directories. Data will be accessible on the Philips HealthSuite Digital Platform, an open and secure, cloud-based IT infrastructure.
"Lumify is a game-changing innovation," said John Bailitz, MD, emergency ultrasound physician and leader with the American College of Emergency Physicians and the Social Media and Critical Care organization, in a Philips statement. "The affordability, flexibility and versatility of Lumify make it appealing to those working in emergency settings."
Data from Lumify will be accessible on the Philips HealthSuite Digital Platform, an open and secure, cloud-based IT infrastructure, allowing clinicians and health systems access to powerful data and analytics to help improve patient care.
In addition to the subscription pricing model, Lumify is also now available through a one-time purchase transaction. It is presently compatible with Android devices, and the company said last year it had plans to make it compatible with Apple devices as well.
The Lumify was born of Philips' work on creating 3-D ultrasound imaging transducer about a dozen years ago, Randy Hamlin, vice president and point-of-care business leader of Philips Ultrasound, told Qmed when Lumify came out last year.
"It's really centered around the ability to put all the electronics in the transducer itself, in the device that couples to the patient," Hamlin said. "That frees you up to then have a CPU and display device at the other end of the wire. For us to achieve that, we needed highly capable electronics in a low-power format, so we just use the power from the smart devices."
The company's vision is to put smart-device ultrasounds in the hands of more professionals in more locations, Hamlin said of the latest device approval.
"With the S4-1 transducer and clinical pre-sets, Lumify is further extending the reach of ultrasound by delivering exceptional image quality, now for routine cardiac exams, and creating better connections between clinicians and their patients," he said.
Nancy Crotti is a contributor to Qmed.
Like what you're reading? Subscribe to our daily e-newsletter.
[Image courtesy of Philips]
About the Author(s)
You May Also Like


