Your 5 Step Blueprint for Great Medtech Design Reviews
April 9, 2015
Design reviews can help your medical device engineers stay on track--if done correctly.
Jon Speer
|
Optimizing the process for design reviews can help product development stay on track, says Jon Speer, cofounder of Greenlight.guru. |
One of my favorite parts of the design control process is design reviews. If you've conducted design reviews of your own, you may think I'm crazy. It is true that, if they're not done properly, design reviews can seem like a huge waste of time, and developers too often hold them just to check a box. Trust me. I've had a few of those myself.
But, once I realized the process for a great design review, it became much easier to see their value. According to the FDA, design reviews should "ensure that formal documented reviews of the design results are planned and conducted at appropriate stages of the device's design development." After a design review, all of the results should be put into the Design History File (DHF).
Many people argue over what the best frequency of design reviews is. There's no clear guideline; the answer is usually, "as often as needed."
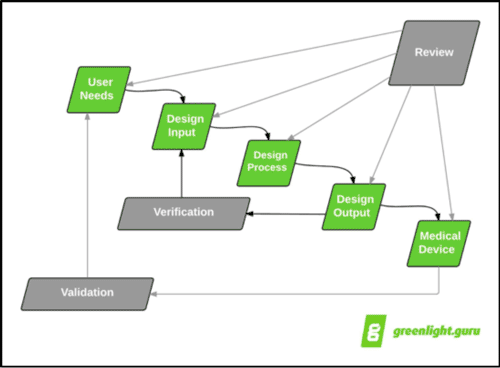
If you take FDA Design Control "waterfall" diagram literally, you would have a design review at the end of every stage of design controls. This may or may not be practical. Mapping out when you'll have design reviews during the design planning phase is a better solution.
|
|
(Caption: Representation of the classic design controls waterfall diagram originally published by Health Canada and published in FDA design controls guidance)
Design reviews are key points in time during a medical device product development project to ensure the efforts are moving in the right direction and on track. Because of this, I will say, you can't really have too many design reviews.
During my years of medical device development, I learned quite a few right (and wrong!) ways of doing design reviews. Here are my top five guidelines to help ensure great design reviews:
1. Get the Right People in the Room
One of my biggest design review mistakes was having the wrong people involved. Or not having the right ones.
Plan on including people from the various functions (engineering, manufacturing, marketing, and so forth) in all the reviews. You also need one person who is independent of the process. This is an FDA requirement and an important piece you can't overlook.
2. Keep Design Reviews to One Hour
Once you have the people, respect their time. Strive hard to limit design reviews to one hour.
This may mean that you break some design controls (like design inputs) into multiple, separate reviews. While this seems wildly inefficient, trust me: it's worth it. You'll get much more complete feedback and review if everyone focuses in for just one hour. Any more than that and you'll lose the audience, missing the point of the review altogether.
3. Be Prepared
Share all the documentation with everyone invited to the design review. Ideally, you should share this a week in advance, but definitely no less than three business days beforehand. Then, contact everyone a day or two in advance and see what questions or comments they may have.
After that, make an agenda, and stick to it! Use the comments you collected to lead off the discussion. If it starts to get off track, make a note, but steer it back to the topic at hand quickly.
4. Have a Form and Checklist
The whole purpose of design reviews is to make sure the device is safe, effective, and appropriate. Besides an agenda, consider having a checklist to ensure you cover all aspects of the design control you're reviewing.
You should also definitely document the event on a design review form. At a minimum, you need to capture the topics discussed, documents reviewed, any action items identified, and list of attendees.
5. Close the Loop
During design reviews, there will be action items identified, which you should capture on your design review form. But it's also very easy to forget about closing these action items out after the design review. This is a big no-no. Always close the loop for all action items.
In summary, design reviews don't have to be stressful or a waste of time. It should always be helpful to touch base and make sure you're meeting design control requirements as you move forward.
Follow the blueprint above, and, I promise, you'll begin to see the benefits, too.
Hear Speer deliver a talk related to medical device quality at BIOMEDevice Boston, on May 7, 2015. |
Jon Speer is the founder of greenlight.guru and a managing partner at Creo Quality LLC.
About the Author(s)
You May Also Like