Compact Displays Feature High Resolution
October 1, 1998


Electronics
Combined Rocker Switch and Circuit Breaker Protects from Water Splashes
Suitable for patient monitoring instrumentation and other equipment
E-T-A CIRCUIT BREAKERS (Mt. Prospect, IL) has introduced a combined rocker switch and CBE (circuit breaker for equipment) to offer full protection from water splashes. The Model 3130 CBE is especially suitable for medical electronics applications where the equipment may be exposed to water or damp conditions, such as during cleaning or sterilization.
The unit is designed for both reliable protection and aesthetic appeal. It conforms to IP65, an IEC standard definition that indicates the degree of protection against water ingress. Specific applications include patient monitoring devices, heart pumps, hospital beds, and paramedic equipment.
Combining a switch and circuit protection in one compact unit, the single-pole version weighs only 0.6 oz. It uses less space and incurs lower product costs than if separate components are used.
The Model 3130 is the first component of this type designed especially for external snap-in panel mounting. Rocker switch illumination is optional. The versatile circuit breaker can withstand temperatures from –30° to 60°C and has undergone extensive testing in a variety of situations.
For more information, contact E-T-A Circuit Breakers at 847/827-7600.

Display Technology
Compact Displays Feature High Resolution
Use thin-film technology
TWO COLOR DISPLAYS suitable for medical instrumentation are distinguished by their small size and by the high resolution they provide. The screens, available in 1.8- or 2.5-in. sizes, use thin-film technology (TFT).
The Models TFT-18DM and TFT-25DM feature 160 x 234-pixel resolution and an analog RGB interface. They measure only 51 x 39 x 13 mm and 68 x 55 x 13 mm, respectively. Both displays come with a CCFL backlight and are available as full monitors with inverter and control circuitry.
Power consumption is 0.90 W for the TFT-18DM and 0.97 W for the TFT-25DM. Both use an external dc/dc converter/inverter. The manufacturer, Purdy Electronics (Sunnyvale, CA), says that the TFT technology provides the displays with high contrast, good response, and an optimal viewing angle. Due to their small size, they are suitable for handheld medical instruments and diagnostic devices.
For more information, call Purdy Electronics Corp. at 408/523-8230.
Disposables
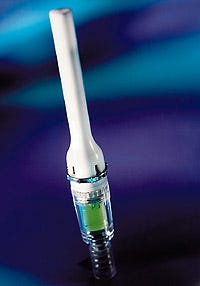
Needlefree Injector Offers Comfort and Safety in Home-Use Applications
Designed to be used by OEMs in developing self-care products
MERIDIAN MEDICAL TECHNOLOGIES INC. (Columbia, MD) has introduced a needlefree disposable injector. The prefilled injector is meant to be used by manufacturers in the development of products that can be self-administered by patients.
"The Intraject system provides patients with a new level of convenience, comfort, and safety, while also reducing total healthcare costs by eliminating the need for office visits for injections," says James H. Miller, Meridian's CEO. Miller notes that the injector offers significant new product development opportunities for existing medications, as well as a practical means of administering the growing array of new pharmaceuticals and biologicals that require injection.
About half the size of a fountain pen, the inexpensive disposable device is fast and easy to use, and causes virtually no pain, according to Meridian. The patient simply removes the safety seal and presses the injector against an arm or thigh. A precise dosage of medication is automatically injected through the skin within 50 milliseconds as compressed nitrogen expands to activate the system.
According to Gerald Wannarka, Meridian's vice president of technology, "The availability of improved technology for self-administration of injectable drugs should significantly improve patient compliance, as well as provide added flexibility in the development of dosage regimes. We believe that the Intraject system has the potential to displace a significant portion of the conventional needle syringe market."
For more information, call Meridian at 410/309-6830.
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
