10 Finalists Announced for 2015 Dare-to-Dream Medtech Design Challenge: pTAV
pTAV Name your device and explain how it works?
September 9, 2015
pTAV
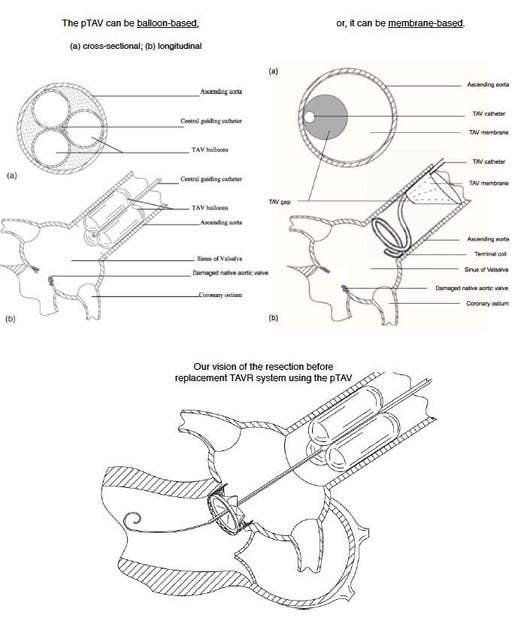
Name your device and explain how it works? Percutaneous Temporary Aortic Valve (pTAV) - is a simple, catheter system designed to be readily inserted in the ascending aorta to sustain a collapsing hemodynamics when the native aortic valve has an acute failure.A temporary aortic valve must provide two essential functions that the native valve serves (both occurring in diastole): to prevent acute aortic regurgitation (AR) into the left ventricle (LV) and to facilitate coronary filling. Clinical use of this device (in endocarditis or valve replacement, see below) mandates the pTAV to be deployed in the ascending aorta. Past attempts to develop a temporary aortic valve have failed due to inability to provide adequate coronary filling. Our pTAV device has a patented built-in gap to provide both adequate coronary filling and significant reduction in AR during acute aortic valve failure. This design has undergone successful prototyping in mathematical, bench and animal models. �What problem in healthcare does the device solve? Immediate application of the pTAV is a bridge to surgical aortic valve repair/replacement (AVR) in endocarditis. In impending hemodynamic collapse due to acute AR from endocarditis, immediate surgery may be unavailable due to active infection or lack of on-site heart surgery. Stabilizing the hemodynamics with the pTAV for just a few hours to a couple of days can be life-saving.The more ambitious goal is to build a fully standalone transcatheter system to replace the aortic valve (TAVR). Surgical AVR is a time-proven surgery with low complications. Its advantage over TAVR is the removal of the diseased, bulky valve before replacement. Despite advances of current TAVR, it is unlikely to replace surgical AVR in the low-risk patients. Our pTAV can allow for the development of a transcatheter valve resecting system. With some of the disease material removed prior to replacement, this new TAVR concept will be competitive with open-heart surgery for the low-risk patients. Why should the device be commercialized? Commercialization phase 1: pTAV for the treatment of aortic endocarditis. The estimated number to treat is relatively small, but the worldwide market is estimated to be 500,000 cases annually with a revenue of $30-50M. Phase 2: resection prior to replacement TAVR system. The uncontested low-risk AVR patients represent 75% of the market, which is forecasted to be in the multi-billions in the coming years. What inspired you to design this device? Analogous to complex percutaneous coronary interventions, current TAVR needs the necessary adjunctive tools to handle the full-spectrum of aortic valve diseases and patient populations. Submitted By Paul Ho |
|
You May Also Like




