Blurred Lines: Big Pharma's On-Again Off-Again Relationship with Medtech
October 6, 2015
The boundaries between the traditional drug and medtech realms are gradually eroding, being redrawn, and then eroding again.
Brian Buntz
|
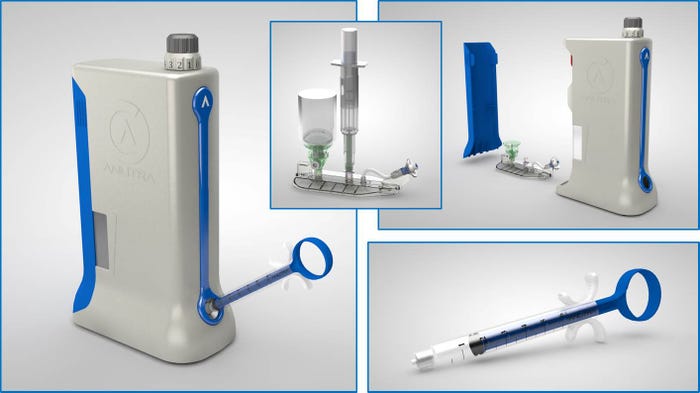
EG-GILERO developed a Local Anesthetic Delivery System for Anutra Medical that launched in 2015. The product was named the most-innovative technology on display at MD&M East. |
"These cycles have occurred in the past where pharma companies gobble up device companies and then spit them out. And occasionally, you'll see a medical device company gradually morph into a pharma company, or vice versa," observes Ted Mosler, CTO at contract manufacturer EG-GILERO (Durham, NC).
Pondering these changes can be dizzying. A little more than a decade ago, Abbott Laboratories announced that was spinning out Hospira. At that time, Abbott was a fairly traditional drug company and Hospira was a hospital-products company.
Then in 2012, Abbott announced that it was spinning out its biopharma business to focus on medical devices. As for Hospira, the company had significantly boosted its pharma business by acquiring Mayne Pharma Ltd. in 2007 and then later Pliva-Croatia and Orchid Chemicals & Pharmaceuticals Ltd. in 2009 and 2010. The company eventually became the single biggest maker of generic injectable pharmaceuticals. Now, the company was recently acquired by Pfizer, one of the biggest pharmaceutical companies.
"Another good example is Cardinal Health, which spun out Carefusion and got rid of their device business," Mosler says. "Then Becton Dickinson stepped in and bought Carefusion." Now you have Cardinal Health buying Johnson & Johnson's vascular device business, Cordis.
And speaking of Johnson & Johnson, that company is gradually whittling away its medical device business to focus on more profitable drugs.
The boundaries between the two businesses began to become unclear with the proliferation of combination products, which comprise approximately one-third of all medical products in development.
In addition, there are medical device companies seeking to develop products that compete head-to-head with drugs. One of the most recent examples in recent memory is that of renal denervation, which promised to be a promising device offering for people with drug-resistant hypertension. Clinical trials have yet to convince the medical establishment of its promise, however.
Other examples of projects that are blurring the lines between the two industries include devices that stimulate the spine or the brain to help treat disorders ranging from chronic pain to Parkinson's. Such device treatments either provide alternative to drugs or can be used in conjunction with drug therapies.
"And then on the pharma side, you have all of this competition for these big blockbusters. Some of the big drug companies are working to prolong their drug patents by developing novel drug delivery devices for those drugs," says Tim Hopper, CMO at EG-GILERO. "If you can get a patient population used to a device, you can sometimes extend the drug patent and make it tougher for the generic drug maker to take your business when the drug does go off patent."
"The other big problem for pharma is patient compliance," says Hopper. "You still can't really track people taking pills." There have been attempts to address the problem--everything from putting sensors in pills to low-tech pill counters. We are seeing more interest in implants that elute a drug for, say, 6 months. "Once implanted, these devices can elute a given drug dose more consistently taking patient compliance out of the equation. We are also seeing drug delivery patches that could help improve compliance as well," Hopper says.
EG-GILERO expects to see more drugs and devices being used not just independently, but increasingly together as part of a system. There are drugs that can improve performances of devices and new drug-delivery devices that can improve patient compliance. "There is a lot of activity in this space," Ted Mosler says.
Learn more about cutting-edge medical devices at MD&M Philadelphia, October 7-8. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like