What the Future Looks to Life Sciences Instrument Makers
October 5, 2015
A recent survey of 302 life science instrument makers and users were given the opportunity to select the technologies that could most benefit their industry. Respondents also weighed in on key trends, including how they are dealing with regulatory and cost pressures.
Brian Handerhan
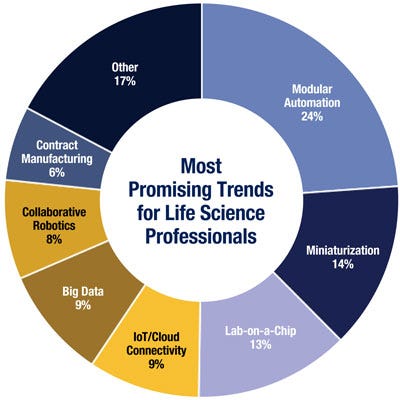 Lab-on-a-chip technologies, modular automation, and miniaturization hold the most promise when it comes to improving life science instruments, according to many of the respondents of a recent Parker Hannifin Life Sciences survey of engineers and business managers in the space.
Lab-on-a-chip technologies, modular automation, and miniaturization hold the most promise when it comes to improving life science instruments, according to many of the respondents of a recent Parker Hannifin Life Sciences survey of engineers and business managers in the space.
Of all of the technologies identified in the survey, lab-on-a-chip (LOC) technology was viewed as having the largest potential to revolutionize the industry. The goal of scaling a single lab process down to a chip format is to reduce fluidic volumes to less than a picoliter, thus downsizing the reagents and specimen samples and their associated costs. LOC also promises to reduce the time needed to obtain test results and boost throughput, thanks to mass parallel processing. This technology is still too new to have reached widespread use, however.
Modular automation was a popular choice, given its ability to reduce overall product development time. Modular automation can be easily reapplied in future designs, allowing for reduced verification, validation, and qualification cycles. These are innovations that have already been through their own verification and validation processes and can therefore accelerate the process of getting an instrument through qualifications. OEM managers can thus attack lower volume applications than could not normally be justified, which creates additional customer value because of their ability to supply more complete laboratory automation solutions.
Survey respondents selected the miniaturization of motion control and fluidics as a promising trend because it can help instrument manufacturers meet the demand for small instruments with a small laboratory footprint. In addition, miniaturization can lead to the development of instruments that consume a minimal amount of reagents as well as instruments that require small test samples. From the end user perspective, these can reduce the overall cost of ownership of an instrument.
Other promising technologies identified in the survey include:
Big Data plays a key role in enabling quantum leaps forward in DNA and molecular technologies. Big Data continues to be a major driver for reducing cycle times and lead to the development of ever-faster sampling technologies.
Collaborative Robotics involve devices that can operate in parallel with humans without the need for safety guards and interlocks because the robotic devices are inherently safe for human interaction.
Internet of Things (IoT) is a network of devices embedded with electronics, software, sensors, and connectivity, enabling it to achieve greater value and service by exchanging data with the manufacturer, operator, and/or other connected devices.
Contract Manufacturing is finding broader use in the production of medical Instruments, leading to an increase in production capacity and flexibility, while cutting overall manufacturing cost.
All of these innovations have been enabled by distributed machine control--a control architecture for reducing cabling and control panel size. Wireless technology has also been leveraged to reduce cabling in instruments. The mass customization manufacturing approach is making low volume production cost effective. Other enablers include engineering outsourcing; servo positioning, the use of brushless DC motors to improve speed, responsiveness, and accuracy; and the creation of a standard communication protocol to simplify communication between instruments and modules within instruments.
Challenges to Progress
The trend toward smaller reimbursements to the laboratory makes the need to reduce costs the number-one challenge.
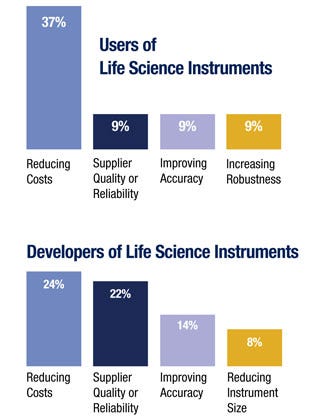 Cost pressure is forcing cost reductions down to the OEM and the component suppliers. Users are looking at the cost model from the per-test and floor-space-per-test perspectives, while OEMs are looking at their bill of material and engineering costs. Many respondents pointed to the concept of total cost of ownership as the critical measure, with a relatively even split looking at it through the eyes of the end user in terms of instrument performance compared to looking at the internal costs of the instrument.
Cost pressure is forcing cost reductions down to the OEM and the component suppliers. Users are looking at the cost model from the per-test and floor-space-per-test perspectives, while OEMs are looking at their bill of material and engineering costs. Many respondents pointed to the concept of total cost of ownership as the critical measure, with a relatively even split looking at it through the eyes of the end user in terms of instrument performance compared to looking at the internal costs of the instrument.
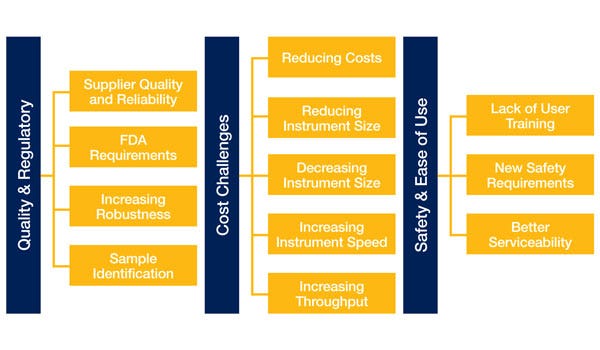
Quality and regulatory challenges highlight the need for "bullet-proof" and "robust" designs, with much discussion about the move away from strictly internal quality measurements like returned parts per million (RPPM) or mean time between failure (MTBF) and looking at more end-user-centric measurements like laboratory uptime. MTBF was seen as a "killer variable."
Instrument safety and usability are related to the increase in instrument complexity and difficulty among laboratories that don't have engineers or maintenance staff to deal with downtime or troubleshooting. With advances in cellular research, multiple instruments are being configured together, with lab robots taking care of loading and unloading. This makes user safety paramount, whether achieved through the use of collaborative robots or simply with machine guarding. Sample safety is another aspect of user safety, and here again, guarding and closed-loop control are needed to keep users safe from in-process samples and to protect samples from possible user interference or tampering.
Other challenges commonly addressed by OEMs and their customers include:
Accuracy also weighs heavily on the quality scale. This requires new levels of performance for motion control systems. Trends toward new technologies like DNA sequencing, digital pathology, cell inspection and manipulation, and molecular diagnostics require precision and repeatability outside of the normal liquid handling or robotic handling applications--thanks primarily to smaller sample sizes.
People problems cited in the survey include lack of engineering manpower and training. Since the recession of 2008-2009, staffs have been kept relatively trim, causing teams to extend project timeliness, outsource design elements, or find subsystem level suppliers that can provide more completely engineered system solutions.
More complex, automated instruments are being introduced into laboratory settings where the staff does not have the expertise or bandwidth to troubleshoot. This makes good user training more difficult and therefore less likely.
On the regulatory side, the discussion ranged from the trend away from releasing instruments outside the United States to synchronizing the instrument revenue stream with gaining FDA approvals. The concern is that both European and Chinese regulations are tightening to the point where approvals in all three markets might happen on the same overall timeline. Design strategies must help shorten the overall verification and validation process so that regulatory approvals will not create a major time barrier to achieving the desired revenue ramp-up.
Other challenges mentioned in these surveys include:
Serviceability: Designing to make instruments more field serviceable to minimize cost and maximize uptime.
Robustness: Designing instruments to maximize uptime and minimize user interaction requirements.
Instrument Size: Reducing the floor space requirements for instruments as a result of constraints within the target lab environment.
Sample Size: Development of processes to minimize the size of samples required to run tests. Samples are considered a precision resource and minimizing the amount required is a consideration.
Engineering Outsourcing: Using outside engineering resources to help with instrument designs.
Differences by Job Function
Across the board, our respondents identified cost, quality, regulatory challenges, safety and usability as their major challenges in the near term. Although engineers tend to have priorities that are different from those of managers (and those differences came across in our surveys), these issues came forth as the megatrends among their challenges.
To slice the results a bit thinner, among technical decision makers, modular automation was given the top ranking among the positive trends. Engineers focused on the modularity concept as a way to accelerate the design cycle of instruments while being able to make design more robust.
The technical decision makers rated reducing costs significantly higher than any other challenge of implementing automation, with the cost concern being squarely focused on minimizing the bill of materials cost. Other challenges prioritized by the engineering respondents were improving accuracy, improving robustness, decreasing instrument size, decreasing sample size, and improving serviceability.
Among business decision makers, modular automation was also identified as the most important positive trend, not only to enable faster instrument development but to allow them to stretch their engineering resources and to generate revenue faster by shortening the verification, validation, and qualification cycles. Contract manufacturing of instruments was next on their priority list because these operations can quickly ramp up capacity to meet highly variable demand curves, position production closer to the center of their customer base, and institute client-compliant procedures and controls. This group identified reducing costs as the most critical challenge being faced. These business decision makers shared the technical focus on bill of material costs but also pointed to the cost of engineering resources, the development cycle time, and the cost of completing all verification and validation as other cost drivers in consideration.
Among influencers, modular automation again was given the highest priority as the most positive trend in improving instrument development and instrument results. This group pointed to the benefit of modular automation as an enabler for providers to broaden their portfolios and attack niche markets, thus improving their ability to provide full laboratory solutions.
Both developers and users of life science instruments pointed to modular automation as the number-one positive trend, again with the focus being on speed to market, robustness, and ease of use.
Users of life science instruments rated reducing costs as the largest challenge by a 4x factor over the next closest ranked challenge. Developers of life science instruments also saw reducing costs as the highest ranked issue, but saw improving accuracy as a similarly rated challenge. The concept of accuracy was aimed at many aspects of performance, which included true accuracy, repeatability, responsiveness, and deterministic positioning.
Conclusion: Lab Technology Advances Driven by Social Trends
Advances in laboratory technology are directly tied to overall trends in the economy, education, and population demographics. The cost of healthcare is among the top budgetary and societal concerns among all citizens and their governments. Professionals in the life sciences are in a unique position, both to drive the dialog among these groups and to affect its outcome. Before they can do that, their hopes and concerns must be addressed.
Bottom line, the promise of modular automation is less related to tactical issues like bill of material cost reduction and more pertinent to business strategies like getting products to market faster and with fewer resource requirements. OEMs are also leveraging modularity to increase the overall robustness of instruments and to get through regulatory-related verification, validation and qualification more quickly. And because modular components are easier to field service and repair, safety, and reliability are a natural outcome.
On the engineering challenges side, it's getting harder for smaller staffs to handle the multitude of projects in their pipeline. That makes outsourcing both an opportunity and a challenge. But modular automation addresses that challenge too by shortening the engineering time to develop new instruments. Not only that, but modular automation suppliers are often capable partners who can help counterbalance light staffing with their ability to design and engineer motion control subsystems for laboratory instruments.
Brian Handerhan is a business development manager focusing on Parker's Life Science Automation group. He can be reached at [email protected].
About the Author(s)
You May Also Like


