Taxus Stent Could Give Boston Scientific Market Leadership
Originally Published MDDI May 2004NEWSTRENDS
May 1, 2004
Originally Published MDDI May 2004
NEWSTRENDS
|
The Taxus Express Paclitaxel-Eluting Coronary Stent System helps prevent the renarrowing of coronary arteries. |
Boston Scientific Corp. (Natick, MA) in early March received FDA approval for its Taxus drug-eluting stent. Industry observers expect this stent to be the most profitable—and perhaps the most significant—device to come to market this year.
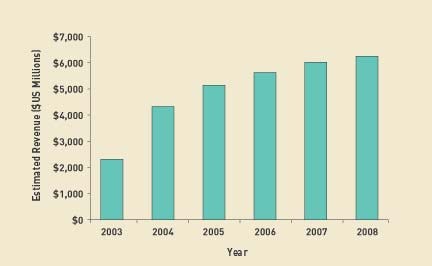
The drug-eluting stent market has been projected to reach more than $4 billion by the end of 2004 and as much as $5 billion shortly thereafter, making it far more lucrative than a typical device market (see Figure 1). Boston Scientific expects sales of Taxus to surpass those of its only U.S. competitor, J&J's Cypher, made by Cordis Corp. (Miami), within months. Many financial analysts share that opinion. Taxus got off to a strong start with U.S. sales of $98 million in March, beating company estimates of $54 million to $75 million.
After deployment in the coronary artery, drug-eluting stents dispense a drug to prevent restenosis, or reblockage. Taxus consists of the Express coronary stent on the Maverick balloon catheter platform. The stent contains the polymer Translute that deploys the drug Paclitaxel. In clinical trials, Taxus users had a 3% rate of target lesion revascularization after nine months, compared with 11.3% for bare-metal-stent patients. Therefore, only 3% of patients with Taxus stents had to be retreated for blockage. In many cases, Taxus was able to prevent restenosis in particularly complex and difficult lesions, said Eric Simso, Boston Scientific's vice president of stent marketing.
|
Simso: Taxus expects to capture 65% of the U.S. market for drug-eluting stents within several months of launch. |
Cypher has demonstrated excellent clinical results, but Boston Scientific believes Taxus can surpass those. The company claims that Taxus is easier to deploy and works better in some classes of patients, such as those with diabetes.
“Clinically, Cypher is a great product, but the feedback we've gotten is that the Taxus Express stent is easier to use,” Simso said. “It's more deliverable and more flexible.” As a result, he said, Boston Scientific believes Taxus can capture 65% of the U.S. market share for drug-eluting stents within several months of launch.
Boston Scientific also expects to have an advantage in supply, Simso said, noting that the company had 200,000 units, in all possible sizes, ready to ship upon approval. “We have all the right sizes and the right dimensions available right away,” he said. “We've gotten to the point where we have yields up to very high levels. We could build enough to satisfy 75% of world demand for drug-eluting stents if needed.”
By contrast, J&J did not have certain sizes available immediately at launch, leading a few doctors to deploy stents that were too small for patients with large lesions, which caused thrombosis in some cases. J&J was also criticized for not manufacturing Cypher stents fast enough to keep up with demand.
Since the Taxus launch, J&J has received good news clinically, but bad news from a manufacturing perspective, about Cypher.
In mid-March, Cordis released four-year findings from Cypher's pilot study and three-year findings from its European and Latin American clinical trial. In the pilot study, the target lesion revascularization rate was 2.8% after four years. In the three-year study, the rate was 5.0%, compared with 14.4% of bare-metal-stent patients.
But on April 1, 2004, Cordis received an FDA warning letter regarding GMP violations in the manufacture of Cypher. Cordis pledged to address all issues raised by FDA and said the action would not affect supply. “All products released to the market for use in patients are safe and meet product specifications approved by FDA,” said Rick Anderson, president of Cordis' cardiology division.
Although early indicators are positive for Taxus, Boston Scientific must keep in mind that results for drug-device combinations can change over time, said Michael Drues, PhD, president of Vascular Sciences (North Grafton, MA). “Getting a handle on the long-term effects of combination products is a challenge for the device world,” he said. “Historically, device clinical trials measure effectiveness right away. Device companies are not used to having to wait one or two years to see if something works over that time.”
Overall, he said the initial research for the Boston Scientific product looks very promising. But there is always the possibility that once it comes to market, problems could develop. “That's just the reality of the business,” Drues said.
Paclitaxel is a reformulated anticancer drug that suspends activity of targeted cells, rendering them unable to enlarge, divide, or secrete. It is licensed from Angiotech Pharmaceuticals Inc. (Vancouver). Translute, the polymer that dispenses the drug, was developed in-house at Boston Scientific and is a proprietary formulation, Simso said. It dispenses the majority of the drug within the first 10 days of deployment, and the rest over the following 20 days.
|
Figure 1. Worldwide revenues from drug-eluting stents are expected to grow at a compound annual rate of 24% over the next five years. Source: Navigant Consulting (www.navigantconsulting.com) (click to enlarge) . |
“The most difficult part of coming up with a polymer was finding one that was vascular-adaptive and did not cause inflammation,” Simso said. “A second concern was finding one that could be sterilized with gamma or EtO and then stay stable over time. And then the polymer had to withstand the stent expanding from as small as 1 mm to as large as 4 mm.”
Another tough task was navigating regulatory approval, which involved not ony CDRH but also FDA's Center for Drug Evaluation and Research.
“It's a huge challenge for device companies to [have to perform] completely new rounds of testing for every batch and to demonstrate that they all fit into the narrow band of tolerance,” Simso said. “Dealing with a product that can expire is also new for us. The volume of test data and the volume of the submission exceeded by fivefold anything we'd done before. There was a lot of give and take, and we learned a lot.”
Patrick Driscoll, president of MedMarket Diligence (Foothill Ranch, CA), said Taxus should succeed if Boston Scientific's track record is any indication. “Boston Scientific has proved itself to be a worthy competitor in the device industry. They know what they're doing,” he said. “I'm sure it will be a good product, based on the fundamentals of how they do business.”
The next step for Boston Scientific, Simso said, is to prepare its new stent recently made available in Europe, the Liberte, for application with Paclitaxel. Liberte is stronger and more flexible than other stents, he said.
Copyright ©2004 Medical Device & Diagnostic Industry
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)

