St. Jude's Nanostim Leadless Pacer Still MIA in Europe
St. Jude Medical is struggling with its leadless pacemaker in Europe, while Medtronic just won CE Mark for its own rival product.
May 15, 2015
Arundhati Parmar
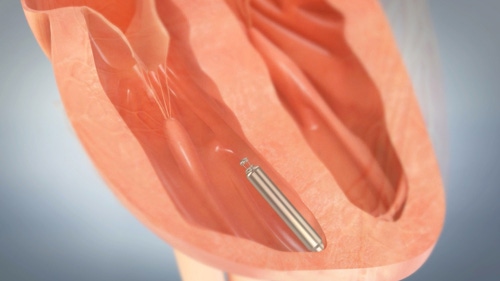
St. Jude Medical was the first in the world to commercialize a leadless pacemaker following its acquisition of Nanostim and the device obtaining CE Mark in 2013.
But the novel technology is not currently being sold in Europe. Last year, a postmarketing study of about 200 patients revealed that there were two patient deaths and six instances of perforation. As a result device sales were halted.
Results show that the adverse events were not related to the device but rather to the technique employed in implanting the transcatheter pacing system, according to a research note from Glenn Novarro, an analyst with RBC Capital Markets. Novarro, who published his findings after meeting with St. Jude executives at the Heart Rhythm Society's annual meeting set to conclude tomorrow in Boston, noted that the Minnesota medical device company is working with European regulators to update its training protocols. The company expects Nanostim to be back in action later this year, but didn't specify an exact timing, noted Joanne Wuensch, an analyst with BMO Capital Market, in a research note.
The relaunch couldn't happen soon enough.
In mid April, Medtronic announced that its rival product - Micra - has obtained CE Mark in Europe. At the HRS meeting, the Irish medtech company published results showing that every single of the first 140 patients who were implanted with the device had experienced a "successful implant procedure." Further, mean electrical pacing measurements at the one-month and three-month follow up were within expected ranges.
"[Medtronic] expects to launch in Europe within the next few months," Novarro wrote. "Given Micra’s clean clinical profile, we see an opportunity for major share gains in the single chamber market in Europe."
Leadless pacemakers use a catheter that can deliver the implant instead of a surgical incision required to place conventional pacemakers and the creation of a pocket under the skin where those older generation pacemakers can reside.
Analysts believe that adoption of leadless pacer technology could be rapid. Both St. Jude Medical and Medtronic are currently conducting pivotal trials to gain approval in the U.S. St. Jude has completed enrollment in its centers in US, Canada and Europe and plans to submit PMA application to FDA by the end of the year. Medtronic's trial is designed to study 780 patients in about 50 centers in 20 countries.
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
Stay abreast of industry trends at the MD&M East Conference, June 9-11 at the Jacob J. Javitz Convention Center in New York |
You May Also Like


