Cleveland Clinic Team 'Navigates' Tricuspid Regurgitation Case
January 3, 2017
Doctors implanted a new valved stent from Navigate Cardiac Structures Inc. into a patient with severe tricuspid regurgitation under a compassionate plea.
Amanda Pedersen
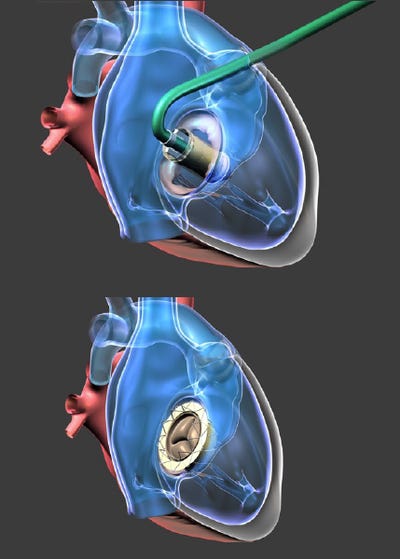
Long and short axis TEE views of the Gate atrioventricular valved stent implanted in the tricuspid valve of a beating heart of swine
A 64-year-old woman with an extensive history of severe tricuspid regurgitation received a new valved stent for the condition through a compassionate plea.
Cleveland Clinic doctors performed the transcatheter procedure, putting in a Gate tricuspid atrioventricular valved stent from Navigate Cardiac Structures Inc.
The Lake Forest, CA-based company licenses the technology from Cleveland Clinic, and the platform also includes percutaneous valve devices for mitral valve replacement. Navigate estimated that the market opportunity for these two conditions is close to $20 billion a year.
As the market for transcatheter aortic valve replacement (TAVR) continues to grow and mature, many cardiovascular companies have taken an interest in transcatheter mitral replacement and repair, as well as the so-called forgotten tricuspid valve.
Navigate said it made modifications to the device that differentiates it from all others that are currently made for atrioventricular valves. The device is designed in the form of a diffuser, or truncated cone, which gives it a low-height profile that can be more easily threaded through the vasculature to reach the atrioventricular valves, allowing it to reside without protrusion into either of the adjacent chambers (atrium or ventricle) for mitral or tricuspid valves.
The company said the valve demonstrated "excellent valvular function, indicating correction of the massive regurgitation problem," after implantation. The patient became stable and her doctors reported at 30 days post-procedure that she is doing well.
"This is a step forward in the treatment of tricuspid regurgitation," said Jose Navia, a Cleveland Clinic cardiovascular surgeon who is also a Navigate scientific advisory board member and a company shareholder.
"The patient's annulus measured 49.7 mm in diameter and there are currently no valved stents that can secure such a dimension without extending into any of the chambers and still provide valvular function," said Samir Kapadia, a Cleveland Clinic interventional cardiologist and Navigate scientific advisory board member. Kapadia noted that there are millions of patients presenting with the same problem.
Navigate is running clinical trials in Chile and Poland of its Navi mitral valved stent for the correction of functional mitral regurgitation, a similar condition. One patient in Chile has just passed the one-year mark and returned to work with a functional valve, the company noted.
Navigate's president and CEO, Rodolfo Quijano, said the company wants to develop devices to replace the lost function of both atrioventricular valves, the tricuspid and the mitral. These devices could be used by cardiologists delivered either by threading through the vasculature, so long as the patient does not have any blood clots in those vessels, or through minimally invasive surgical and beating-heart techniques by a surgeon when the vasculature method is not an option, Quijano said.
The attractive market opportunity for transcatheter mitral and tricuspid repair and replacement devices has drawn a mixture of large and small companies to the space.
Edwards Lifesciences Corp. recently agreed to acquire Or Yehuda, Israel-based Valtech Cardio Ltd. for $340 million, plus up to $350 million in additional milestone payments. Edwards is also developing the Pascal transcatheter mitral valve replacement (TMVR) system and is expected to initiate a CE mark study for that device this year.
Abbott Laboratories also has a horse in the TMVR race with technology inherited through its 2015 acquisition of Tendyne Holdings Inc. and through an investment in Cephea Valve Technologies Inc., of Santa Cruz, CA.
Medtronic also entered the TMVR space in 2015 through its $458 million acquisition of Redwood City, CA-based Twelve Inc.
Amanda Pedersen is Qmed's news editor. Reach her at [email protected].
[Image credit: Navigate Cardiac Structures Inc.]
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
