CDRH Shows Impatience with Defibrillator Issues
CDRH has decided to intervene with external defibrillator problems and recalls that have increased over the past few years. Letters to defibrillator manufacturers were sent in November advising them that FDA “may, in the future, take regulatory steps to improve the current premarket and postmarket regulatory processes associated with external defibrillators.”
December 22, 2010
 The center said it may also begin pre- or postclearance inspections in certain circumstances to help improve defibrillator safety. The increased attention is part of a new defibrillator improvement initiative that seeks to foster the development of better-performing external defibrillators and to address current industry practices for designing and manufacturing the devices.
The center said it may also begin pre- or postclearance inspections in certain circumstances to help improve defibrillator safety. The increased attention is part of a new defibrillator improvement initiative that seeks to foster the development of better-performing external defibrillators and to address current industry practices for designing and manufacturing the devices.
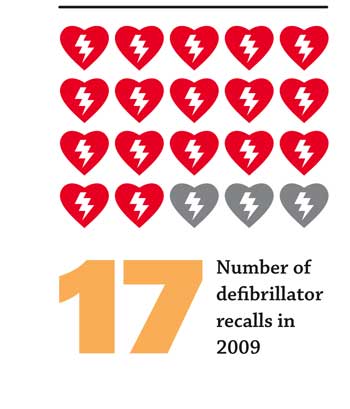
CDRH’s letter said that over the past five years, there have been persistent safety problems with all types of external defibrillators, across all manufacturers of these devices. From January 1, 2005, to July 10, 2010, there were 68 recalls, exhibiting an increase from 9 (in 2005) to 17 (in 2009, the last complete year for which data are available). During this period, FDA received [more than] 28,000 medical device reports (MDRs), which also exhibited an increase from 4210 (in 2005) to 7807 (in 2009, the last complete year for which data are available). FDA conducted multiple inspections of all external defibrillator manufacturers throughout this time period.
The identified problems are preventable and correctable, and they affect patient safety, CDRH said. “We have concluded that there are numerous problems with industry practices for designing and manufacturing defibrillators, handling user complaints, conducting recalls, and communicating with users,” the letter said. “Our analysis demonstrates that there are problems with how well firms control how they purchase and accept components used in the manufacture of external defibrillators. The analysis also demonstrates problems with how changes to the device are evaluated before being implemented.”
CDRH recommends that external defibrillator manufacturers meet with reviewers during the device development process to discuss submissions involving their new devices or changes to existing ones that require significant engineering testing or animal or clinical studies. “Such meetings could include discussions of any studies or testing necessary to support the submission, and any other premarket data needs,” it said. Defibrillator makers should also have early discussions with CDRH’s Office of Compliance on adequate design and purchasing controls.
In mid-December, FDA held a public workshop to discuss external defibrillator innovation and to articulate the agency’s current concerns.
About the Author(s)
You May Also Like


