BioVentrix Resumes Pivotal Trial That Was Interrupted by COVID-19
San Ramon, CA-based BioVentrix is developing a hybrid transcatheter device for left ventricular remodeling after a heart attack. The pivotal trial for the device was originally scheduled to be completed by the end of the year. Now the trial is on track to be wrapped up by 1Q21.
September 7, 2020
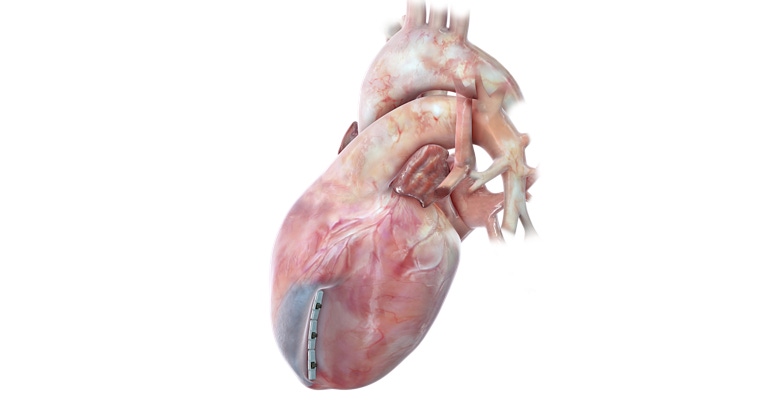
BioVentrix is resuming the ALIVE pivotal trial of the Revivent TC Transcatheter Ventricular Enhancement System. The San Ramon, CA-based company put the 120-patient trial on pause a few months ago because of the pandemic.
ALIVE is set to be completed at the end of 2Q21 but was originally supposed to wrap up by the end of the year, said Ken Miller, BioVentrix CEO.
“COVID-19 did have an impact,” Miller told MD+DI. “Clinical trials took a pause everywhere – not only for BioVentrix but for all of the companies and all products and for the most part elective surgeries took a pause.”
In a release BioVentrix said the first patient procedures completed since elective procedures were paused due to COVID-19 took place at St. Luke’s Hospital of Kansas City, Missouri, and at the Cardiovascular Institute of the South in Houma, Louisiana.
He added, “we’ve had two very successful cases at two separate centers over the last couple of weeks. We’re anticipating additional ones this month as well as significant uptake in 4Q20.”
The trial will have up to 25 sites with a primary endpoint analysis at one year. The trial endpoints include positive effects on volume reduction, ejection fraction, quality of life, New York Heart Association (NYHA) class compared to baseline, exercise capacity, and rehospitalization.
The firm’s Revivent TC System is used in the Less Invasive Ventricular Enhancement (LIVE) procedure, to exclude scar tissue on the left ventricle that has resulted from a heart attack, so the healthy portion of the heart can function more efficiently. Micro-anchors are implanted and designed to remodel the heart to a more normal shape and size and reduce wall stress, which has the potential to improve blood flow throughout the body.
BioVentrix won breakthrough device designation for the Revivent TC System in November 2019. The company said the expectation is to win FDA approval in mid-2022.
The company won CE mark for the Revivent TC System in 2016 and has reimbursement for the technology in Germany.
Another huge milestone the company has its eyes set is an initial public offering in the middle of next year.
“Our plan is to complete the IDE and shortly thereafter take the company public,” Miller said.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
