Ancora Takes on FMR and Heart Failure with AccuCinch
Ancora Heart said FDA has approved an expansion of the company’s feasibility study to evaluate the AccuCinch Ventricular Repair System designed for the treatment of heart failure and functional mitral regurgitation (FMR).
May 15, 2018
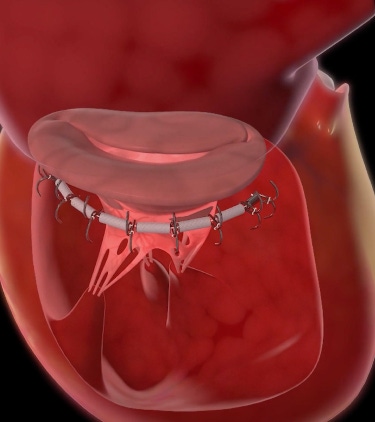
Ancora Heart is making a play for the functional mitral regurgitation (FMR) treatment market – which is currently populated by medtech juggernauts Abbott Laboratories and Edwards Lifesciences.
The Santa Clara, CA-based company came closer to its goal to participate in the market after it received FDA approval to expand the enrollment for an early feasibility study of the AccuCinch. Ancora Heart said it can now enroll a second group of patients and expand to 15 heart centers across the U.S.
“The AccuCinch System is a left ventricular repair technology and it really is a new category and class of therapy,” Jeff Closs, president and CEO of Ancora Heart, told MD+DI. “In the traditional FMR market technology is all focused on the valve, and we know the fundamental problem is the left ventricle is dilated and it has changed the geometry of the valve. Our approach is to target the fundamental cause of FMR, which is a dilated dysfunctional left ventricle.”
AccuCinch is a transfemoral access catheter-based approach. The device can realign the geometry of the valvular apparatus, so it can function more efficiently.
“Our goal in the U.S. is to continue to enroll in that study, which the primary endpoint or primary completion is FMR,” Closs said.
The company said early findings show Ancora Heart’s technology could have some benefit for treating heart failure patients.
“We’ll be submitting an additional protocol to FDA very shortly that will focus on heart failure,” Closs said. “Not too far after that, we’ll submit another protocol to FDA focusing on patients who have had a prior mitral valve intervention that’s reoccurred because the left ventricle had to continue to dilate.”
The company could file for a pivotal IDE sometime in 2019.
“When you think about the heart failure treatment algorithm, patients get on medical therapy and eventually if it’s required they’ll have a CRT,” he said. “And after that you’re really out of options for a heart failure perspective. You have this long kind of clinical chasm or gap before you end up with an LVAD or a transplant. Really what we’re looking to do is target that big clinical gap between a CRT and a transplant.”
FMR Market Players
Ancora Heart could face off against stiff competition in the FMR market.
Abbott is seeking to gain FDA approval for an FMR indication for its MitraClip. The company completed its clinical trial for this indication and it is speculated the firm could present data at the upcoming Transcatheter Cardiovascular Therapeutics meeting in September. Irvine, CA-based Edwards competes in the FMR market with the Cardioband device. Edwards gained access to Cardioband through its $340 million plus milestone payments acquisition of Valtech Cardio Ltd. Cardioband has had CE mark since 2015.
Last month, Cardiac Dimensions made a serious play for FMR and announced it raised $39 million to build strong clinical evidence for its Carillon Mitral Contour System.
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)

