One Specialty? Not for This Device Company
While most small medical device companies focus on one disease area, PAVmed is building a product portfolio that spans several fields and optimizes treatment using approaches from other specialties.
August 23, 2017
Marie Thibault

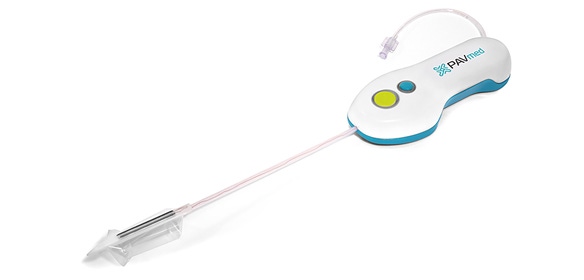
CarpX, designed to treat carpal tunnel syndrome, is one of the devices in PAVmed's product pipeline.
Sitting in his quiet office high above the scurry and scramble of Manhattan's 42nd Street, Lishan Aklog, MD detailed the ways PAVmed, the medical device company he cofounded and leads, differs from other young device companies.
For one, the company's devices don't target just one medical specialty. Unlike many relatively young device firms, PAVmed is a public company. And, though the company has built its product pipeline mainly in-house, it has shown it is eager to look outside for new technologies.
Aklog and his cofounder, Brian deGuzman, MD, aren't new to the device business. In 2007, the two men and Michael Glennon, who is now PAVmed's vice chairman, founded Pavilion Holdings Group. Vortex Medical, Inc., which was the first company in the Pavilion Holdings Group portfolio, commercialized the AngioVac system for removing intravascular clots. Angiodynamics, Inc. purchased Vortex Medical in 2012.
While both Aklog and deGuzman trained and practiced as cardiovascular surgeons, PAVmed isn't a cardiovascular device company. Rather, the founders' training informs, but doesn't limit, product design. None of the six devices that are part of the product pipeline described on the company's website are intended specifically for cardiovascular therapies. Yet elements of some of the devices' working mechanisms echo therapies used to treat cardiovascular diseases. For instance, CarpX, PAVmed's device designed to treat carpal tunnel syndrome, uses a percutaneous approach like that seen in minimally invasive therapies like transcatheter aortic valve replacement.
|
Lishan Aklog, MD |
A Solution Inspired by Another Field
As Aklog tells it, CarpX came about because a distributor who represented the AngioVac system was struggling with the painful symptoms of carpal tunnel syndrome. She asked Aklog and deGuzman for advice before seeking surgery, and that awakened the founders to the clear drawbacks of the therapy options. Neither open surgery nor an endoscopic approach are perfect solutions because of the long recovery times associated with an open surgery and the perceived potential for complications with an endoscopic procedure. Aklog said he was surprised to learn that in the 20 odd years since he'd scrubbed in on a carpal tunnel procedure, the approach hadn't changed.
CarpX's approach of using a percutaneous cutting balloon, making tiny incisions in a patient's skin and threading the balloon into place under the targeted ligament, borrows from therapies already popular for interventional cardiology and peripheral artery disease applications.
During the procedure, a not-yet-inflated balloon is guided under the transverse carpal ligament. The balloon is inflated, bringing the device's bipolar electrodes into contact with the ligament. The electrodes, which are coated to allow for just a small edge of concentrated radiofrequency (RF) energy, deliver two seconds of RF energy at the push of a button. The mechanism allows for RF cutting--not ablation--in a static position, without a back-and-forth motion, Aklog notes, simplifying operation and removing the need for moving parts.
Handling a prototype of the CarpX device, Aklog points out its key features and the crucial considerations that went into the design. For instance, the balloon serves a few purposes. It generates countertension, making it easier to cut the ligament. It acts as a spacer, keeping other components, like the median nerve, away from the cutting mechanism. Lastly, since the intact transverse carpal ligament creates a waist on the balloon that disappears once the ligament is cut, the balloon makes it easy to visualize whether the ligament has been successfully severed.
In addition, the electrodes are radiopaque, making them easy to view during imaging, and the CarpX device includes a nerve stimulator that can be used to test that the median nerve isn't nearby during the cutting step.
The CarpX device has been shown to work reliably on over 30 cadavers, Aklog said. PAVmed is in the presubmission process with FDA for the device and is conducting verification and validation testing. Aklog hopes to submit a 510(k) application for CarpX by the end of the third quarter, with potential clearance anticipated in 2018.
Aklog believes the device could improve the experience of carpal tunnel surgery for patients suffering today. He points out that while approximately 600,000 patients get surgery each year, another one million to 1.5 million people don't pursue surgery. He added that he has "strong faith that a good product"--one that fulfills an unmet need and benefits the patient--will enjoy commercial success.
The Device on the Horizon
While potential regulatory clearance and commercial entry isn't that far away for CarpX, another PAVmed device, the PortIO, is slated for market earlier. PortIO is a vascular access device that is implanted within the bone. This gives clinicians a way to administer medications or fluids into the patient's bone marrow cavity. Importantly, the device doesn't dwell within a vein, cutting the risk of infections and clots. It could also be a good option for patients who have limited or no central venous access, which is not uncommon with severely ill patients.
PortIO has been submitted to FDA via the 510(k) clearance pathway, with an initial indication for use up to 24 hours. This is because that is the indication for the intraosseous devices that might be considered substantially equivalent predicate devices, like those used in trauma and emergency situations. Aklog said the company hopes to secure this indication with FDA clearance and then eventually expand that indication to cover longer term use.
On an August 17 business update call, Aklog told investors that the company is preparing a formal response to FDA feedback on the PortIO submission and will consider the de novo 510(k) pathway if needed. "Although we remain optimistic that, despite the delays, we will succeed in receiving 510(k) clearance for PortIO, the de novo 510(k) pathway is available to us as a backup option, and we are preparing for that contingency," he said. He also noted that the company is considering indications for in-hospital patient use for up to a week and use in dialysis patients.
Evaluating Novel Technologies Outside the Company
While much of PAVmed's product pipeline was conceived of internally, the DisappEAR antibiotic-eluting resorbable ear tubes represent another part of the company's business model. The design for the ear tubes, intended for children with recurring ear infections or fluid buildup, is based on a technology for processing aqueous silk. In November 2016, PAVmed signed a licensing agreement with a group from Tufts University, Massachusetts Eye and Ear Infirmary, and Massachusetts General Hospital. The agreement gave the company the right to develop and sell the DisappEAR ear tubes. At this point, the company is targeting a 2018 FDA submission date for the product.
The resorbable design means a procedure to remove ear tubes, performed under general anesthesia, would not be needed. The post-treatment antibiotic regimen, which Aklog pointed out can cost a few hundred dollars on its own, would also be dropped because of the antibiotic-eluting design. The company estimates the annual market opportunity for DisappEAR could be $200-$300 million.
The DisappEAR agreement sets out a timeline for commercialization milestones. The other parties will earn royalties and will have the opportunity to participate in potential future transactions, such as a sale, Aklog explained.
Aklog hopes the DisappEAR licensing agreement can serve as a template for future agreements, noting that PAVmed was structured to make it "easier to bring in new ideas." The company's management team is evaluating one to five pitches each month and is focused on interventional, single-use technologies.
PAVmed also bucked convention when it became a public company in April 2016 despite being a relatively small, pre-revenue medical device company. Being a public company "gives us access to public capital," Aklog said, adding that this capital enables PAVmed the potential to grow through acquisitions.
The company's stock performance was particularly impressive following the offering, but has lagged in recent months. Acknowledging this, Aklog said that investors are eagerly awaiting meaningful milestones, including clearance of PortIO. The company also raised financing recently, including $5 million in senior secured notes and Series S warrants.
Thanking the company's long-term investors on the August 17 conference call, Aklog said, "[we] look forward to justifying that commitment over the coming quarters for the benefit of all of our shareholders."
Marie Thibault is the managing editor at MD+DI. Reach her at [email protected] and on Twitter @MedTechMarie.
[Images courtesy of PAVMED INC.]
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
