Medtech Startup Showdown 2015: Round 2—Briteseed vs. Interrad Medical—SecurAcath
Briteseed vs. Interrad Medical—SecurAcath
March 31, 2015
| vs. | ||
|
|
|
|
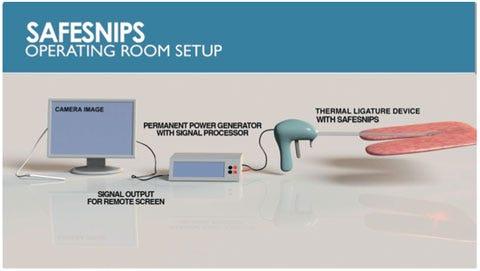
Describe your device and how it will benefit healthcare. | The SafeSnips patented, intraoperative platform technology can be integrated into existing surgical cutting tools to facilitate the real-time detection of blood vessels before surgical cuts are made. U.S. hospitals lose billions of dollars in unreimbursed costs due to inadvertent cuts into vasculature each year. Patients who suffer from these cuts face a mortality rate of up to 32%, and those who survive face an increased hospitalization time of nine days and $210,000 in added costs of care. |
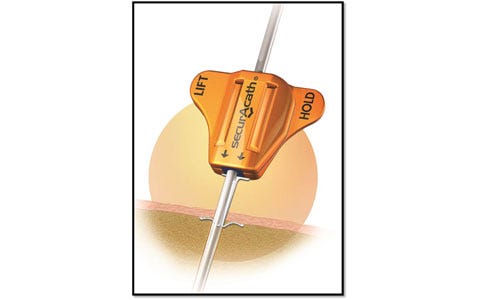
| SecurAcath is a new method for catheter securement that does not require adhesives or sutures. The unique design of the SecurAcath secures right at the catheter insertion site using a small, blunt anchor that deploys in the subcutaneous tissue just beneath the skin. The device decreases the total cost of patient care by reducing complications associated with catheter securement. The SecurAcath dramatically reduces catheter migration and dislodgement, decreases catheter replacement costs, improves efficiency, and allows 360-degree site cleaning while secured. Improved stability combined with better site cleaning may reduce catheter-related infections. |
How does your product differ from the competition? | To date, no company has seamlessly integrated blood vessel detection technology into existing cutting tools. Instead, to locate a vessel during surgery, a surgeon must use a separate laparoscopic Doppler probe or perform intraoperative imaging, such as CT or MRI. All of these alternatives require added steps, which lengthen surgery times and thereby increase the risk of complication. Further, Doppler technology requires surgeons to alter their behavior while imaging alternatives are costly, require contrast agents, and can result in exposure to radiation. |
| The SecurAcath subcutaneous catheter securement technology has many benefits compared with adhesive devices or sutures. SecurAcath offers a low catheter dislodgement rate, which dramatically decreases catheter replacement costs. It decreases the time required to secure, maintain, and remove catheters. The SecurAcath lasts the life of the catheter and, unlike all adhesive securement devices, does not need to be replaced at least weekly. The device design allows for improved catheter site cleaning and minimizes catheter movement, which may reduce catheter-related infections. SecurAcath is also sutureless, eliminating the potential for costly needle stick injuries that can occur when suturing catheters. |
Do you have customers yet? | We are at the prerevenue stage. For future clinical testing, we have identified and will engage the chair of the department of surgery and Loyal and Edith Davis professor of surgery Dr. Nathaniel Soper of Northwestern University to participate as a trial investigator. We will focus on out-licensing this single-use technology across a platform of applications. Initial target customers will be manufacturers of thermal ligature devices (a $1.2-billion global market) and end users will be surgeons who perform minimally invasive general and gynecological surgeries. We have already had partnership and licensing discussions with four major medical device companies, including Covidien, Intuitive Surgical, and Novadaq. |
| The device has FDA and CE clearance and is being sold in the United States, Canada, and Europe.
|
How much money have you raised? | $46,250 in cofounder contributions; significant business plan awards, including $73,500 in cash; $1.15 million raised in seed round using a convertible note |
| Approximately $25 million
|
Who are your investors? | Lead investors include the Evansville, Indiana, Angel Invstors and Grand Order of Successful Entrepreneurs (supported by Dr. Jack Gill, cofounder of Vanguard Ventures); other investors include physicians who would serve as potential end users and domain experienced investors who have spent more than 40 years in the medical device space.
|
| Angel investors |
What is the next milestone for your device? | Refine the current prototype for a pilot study planned at Northwestern University in support of FDA 510(k) application. This includes miniaturization of the SafeSnips technology to fit within a 5-mm trocar, which is expected later this year. |
| Sales revenue growth |
You May Also Like