Medtech Startup Showdown 2015: Round 1—GaitTronics Inc. vs. Interrad Medical—SecurAcath
GaitTronics Inc. vs. Interrad Medical—SecurAcath
March 23, 2015
| vs. | ||
|
|
|
|
Describe your device and how it will benefit healthcare. | SoloWalk is a patient-handling robot that helps frail patients to mobilize after a major illness or injury, such a stroke or hip fracture. The device allows a single caregiver to mobilize a frail patient as opposed to requiring two or more caregivers. Additionally, SoloWalk provides complete automatic fall protection, eliminating the need for manual support from the caregiver if the patient loses their balance. This results in significantly improved safety for patients and caregivers. Overall, these benefits greatly facilitate increased patient mobility which has been shown to improve patient outcomes, including reductions in mortality, delirium, pain levels and length-of-stay. |
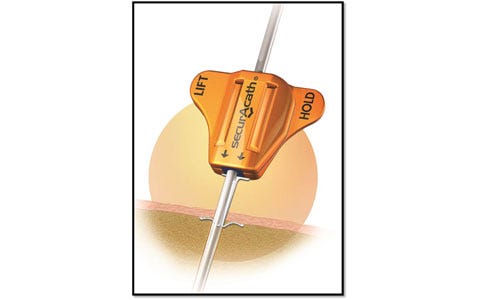
| SecurAcath is a new method for catheter securement that does not require adhesives or sutures. The unique design of the SecurAcath secures right at the catheter insertion site using a small, blunt anchor that deploys in the subcutaneous tissue just beneath the skin. The device decreases the total cost of patient care by reducing complications associated with catheter securement. The SecurAcath dramatically reduces catheter migration and dislodgement, decreases catheter replacement costs, improves efficiency, and allows 360-degree site cleaning while secured. Improved stability combined with better site cleaning may reduce catheter-related infections. |
How does your product differ from the competition? | Current approaches to mobilizing frail patients in an acute care setting consist of using at least two caregivers (often up to four) with the assistance of a platform walker or transfer lift. This approach is very staff-intensive and comes with high risk of injury for patients and caregivers. The increased automation with SoloWalk's robotic system allows a single caregiver to perform the same task with significantly reduced risk of injury. |
| The SecurAcath subcutaneous catheter securement technology has many benefits compared with adhesive devices or sutures. SecurAcath offers a low catheter dislodgement rate, which dramatically decreases catheter replacement costs. It decreases the time required to secure, maintain, and remove catheters. The SecurAcath lasts the life of the catheter and, unlike all adhesive securement devices, does not need to be replaced at least weekly. The device design allows for improved catheter site cleaning and minimizes catheter movement, which may reduce catheter-related infections. SecurAcath is also sutureless, eliminating the potential for costly needle stick injuries that can occur when suturing catheters. |
Do you have customers yet? | GaitTronics is currently partnering with the Ottawa Children's Treatment Centre to pilot the use of SoloWalk with adolescent cerebral palsy patients. We are also working with several hospitals to initiate pilots in an acute care setting in 2015. |
| The device has FDA and CE clearance and is being sold in the United States, Canada, and Europe. |
How much money have you raised? | Since founding in 2012, GaitTronics has raised $400,000 in nondilutive funds, including founder's investment, grants, and entrepreneurial awards. |
| Approximately $25 million |
Who are your investors? | The three cofounders, Dr.Aliasgar Morbi, Dr. Richard Beranek, and Dr. Mojtaba Ahmadi. |
| Angel investors |
What is the next milestone for your device? | GaitTronics is currently working toward initiating pilot studies in acute care hospitals, completing a production model of SoloWalk by early 2016, and receiving FDA approval by mid 2016. |
| Sales revenue growth |


You May Also Like