The Medical Device Market in China
Demand for quality healthcare from a growing middle class and aging population will continue to create opportunities for medtech entrepreneurs.
June 18, 2013

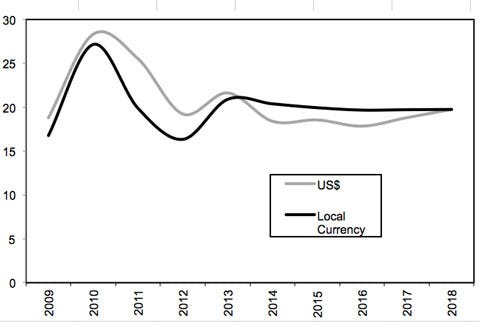
The world’s most populous country, China has been registering double-digit growth in healthcare for several years, even as the markets in developed economies have slowed. It is projected to grow to $11.4 billion by 2015, according to UBM Canon research. This is still small in absolute numbers compared with the United States ($160 billion) and Europe ($115 billion), but China will continue to outpace other countries and reach near parity with the developed nations of Europe by 2020, according to PwC China.1 Medtech market growth is forecast to be in the region of 20% through 2018, according to BMI Espicom (Figure 1).
|
Figure 1. Shown is the percent change in the Chinese medical device market from 2009 to 2018. Source: BMI Espicom |
A $120 billion healthcare stimulus initiated in 2009 has spurred investment in the country’s healthcare infrastructure, creating tremendous business opportunities for manufacturers of everything from consumables to imaging systems. The overarching objective of the stimulus and associated programs is to achieve universal healthcare for China’s citizens by 2020.
Although the country already has made great strides in expanding access to medical services, annual per capita healthcare spending is only $432, representing 5.2% of GDP, according to the World Health Organization. (Per capita spending on medical devices in 2013 is projected to be a mere $12.6, according to BMI Epsicom.) Overall healthcare spending will rise to 7% of GDP over the coming years, as China spends more on drugs, medical devices, and hospital treatments, according to data from McKinsey & Co.
Long regarded as the world’s factory, China is wrestling with rising labor and shipping costs that have eroded some of its competitive edge in international markets. Consequently, the medtech industry has been encouraged to move up the value chain and invest in R&D. Chinese medical device companies such as Mindray and Time Medical Systems, which have performed well in the international marketplace, are held up as examples to follow.2
Regulatory Climate
China’s State Food and Drug Administration (SFDA) regulates the medical device industry and is roughly equivalent to FDA. All imported devices must be registered by the agency. The Ministry of Health is responsible for public health policies, laws, and regulations.
According to consultant Sonia Zhao, companies that have CE or FDA approval should not have regulatory difficulties in placing their products on the market in China. However, other industry observers have characterized SFDA as being unnecessarily complicated and opaque. Recent developments suggest that regulators are seeking to make the approval process in China less burdensome, says Bryan Gilburg, vice president of business development at Emergo Group. He cites as examples the exemption of a large number of devices from the China Compulsory Certification Mark program, the elimination of a step in the registration process, and a proposed expedited review process for innovative medical devices.
Challenges
While China’s medical device industry has made progress in recent years, it still faces difficulties.
Changing Economic Conditions. Salaries are rising and China's overall economic growth, while still healthy, no longer reaches stratospheric levels.
Opaque Regulatory System. Despite some improvements, the approval process in China still has too many steps and takes too long, according to Lin Xianyong, director of SFDA’s medical device safety supervision division in Shanghai. There is no clearly defined evaluation and approval responsibility or well-established supervisory mechanism. The emphasis is on evaluation rather than supervision and rights rather than responsibilities, he notes.3
A Copycat Business Culture. It is rare to see exciting products from domestic companies, notes an analyst from JP Morgan reporting on domestically made products on display at a recent medical product exhibition. “Instead, most products, including patient monitors and ultrasound devices, are indistinguishable from one other,” he observes.4
Pricing Pressures. Fierce competition among domestic players in the low end of the market has eroded prices and profit margins, notes JP Morgan analyst Sean Wu.4
Inadequate IP Protection. Although great strides have been made and medical device manufacturers are at less risk of IP complications than other industries, the perception and, indeed, reality of a “Wild West” mentality remains.
Opportunities
|
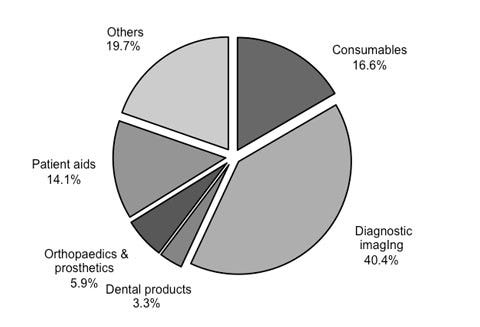
Figure 2. Diagnostic imaging devices were expected to account for the largest share of the Chinese medical device market this year. Source: BMI Espicom |
Despite the challenges, China still offers a wealth of opportunities for medical device manufacturers.
An Underserved Market. In 2012, the Chinese middle class numbered 474 million people, about 36% of the total population. This population will drive demand for sophisticated medical technology. The Chinese medical device market is largely supplied by imports or products made locally by multinational joint ventures, especially at the higher end of the technology scale. If regulatory reforms prove successful and the inadequacy of rural healthcare is effectively addressed, then the sheer number of previously untapped consumers makes China an attractive proposition, according to BMI Espicom.5
Demand for Imports. During BMI Espicom’s most recent study period, imports of medical equipment have grown 20.8% through 2012.6 Diagnostic imaging is expected to account for 40.4% of the medical device market in 2013, according to the firm’s latest data (Figure 2). Consumables represent 16.6% of the market, followed by patient aids (14.1%) and orthopedics (5.9%). Seventy percent of high-end medical devices are imported, according to Hua Yutao, director of China Biology
Technology Development Center. Only about 2000 companies in China produce Class III devices, Yutao said at a CEO summit in Shanghai.6
Expanding Middle Class. Increasing affluence has led to Western-style lifestyles and associated diseases. The cardiac surgery device market, for example, is forecast to grow at a CAGR of 13% through 2018. “The increasing integration of high-fat diets into Asian cultures is leading to an increase in coronary artery bypass graft procedures,” according to Kamran Zamanian, president and CEO of iData Research.7
Targeted Growth. Medical imaging, patient monitoring, in vitro diagnostic (IVD) technology, and high value consumables are the sectors with the greatest growth potential in the coming years. There are 7100 hospitals at the county level in China, only 800 of which have MRI equipment, according to Time Medical Systems CEO Ma Qiyua, adding that imaging companies face a historic opportunity as China upgrades its medical infrastructure.6 Equally attractive from an investment perspective is
China’s IVD Market. Currently valued at more than $4.5 billion, it doubles every three years, according to Whitney Research analyst Nat Whitney. “It will surpass the United States to become the world’s largest IVD market within the next 10 to 15 years," he predicts.8
Conclusion
By 2025, China will have nearly 300 million senior citizens requiring medical services, and its middle class already surpasses the entire U.S. population. Demand for access to quality healthcare and the technology that makes it possible will grow accordingly. Although local manufacturers are increasingly moving up the value chain and developing and manufacturing more sophisticated medical devices, significant opportunities still exist in the Middle Kingdom for entrepreneurial and innovative medical device companies of all sizes.
References
1.Medical Technology Innovation Scorecard, [online] (New York City: PwC, 2011); available from Internet: www.pwc.com/us/en/health-industries/health-research-institute/innovation-scorecard/index.jhtml.
2. Helen Zhang and Norbert Sparrow, “Medtech in China: Transitioning from the World’s Workshop to Innovation Hub,” in Medical Device & Diagnostic Industry [online] April 11, 2011; available from Internet: www.mddionline.com/article/china-workshop-innovation.
3.Helen Zhang, “SFDA Official Makes Candid Comments about China’s Medical Device Regulations,” in medtechinsider [online] October 8, 2012; available from Internet: www.medtechinsider.com/archives/29096.
4.Helen Zhang, “China’s Medical Device Expo Highlights Industry’s Strengths and Weaknesses,” in medtechinsider [online] May 1, 2012; available from Internet: www.medtechinsider.com/archives/27753.
5.BRICS Medical Device Market Reports, [online] (New York City; Espicom Business Intelligence); available from Internet: http://www.espicom.com/bricsm.
6.Helen Zhang, “Medtech Summit in Shanghai Touts Fertile Investment Opportunities,” in medtechinsider [online] June 13, 2012; available from Internet: www.medtechinsider.com/archives/28145.
7.Kamran Zamanian, “Cardiology: China’s Impact on Market Demand,” in Medical Device & Diagnostic Industry [online] June 28, 2012; available from Internet: www.mddionline.com/article/cardiology-china’s-impact-market-demand.
8.Nat Whitney, “Boom Times for China’s IVD Market,” in IVD Technology [online] May 22, 2013; available from Internet: www.ivdtechnology.com/article/boom-times-china’s-ivd-market.
Norbert Sparrow is the editor in chief of European Medical Device Technology. Reach him at [email protected].
You May Also Like